Difference between revisions of "Urothelial carcinoma"
| Line 41: | Line 41: | ||
*These lesions lack papillae and are typical flat. | *These lesions lack papillae and are typical flat. | ||
*Clinically, it may not be possible to differentiate renal pelvis urothelial carcinoma and [[renal cell carcinoma]]. | *Clinically, it may not be possible to differentiate renal pelvis urothelial carcinoma and [[renal cell carcinoma]]. | ||
*May be a part of [[Lynch syndrome]]. | |||
Prognosis: | Prognosis: | ||
Revision as of 02:40, 22 July 2014
| Urothelial carcinoma | |
|---|---|
| Diagnosis in short | |
|
| |
| Synonyms | urothelial cell carcinoma |
| Subtypes | microcystic, glandular, inverted (growth pattern), nested. papillary (dealt with separately in high-grade papillary urothelial carcinoma and low-grade papillary urothelial carcinoma), others |
| LM DDx | urothelial carcinoma in situ, metastatic carcinoma (prostate carcinoma, colorectal carcinoma), inverted urothelial papilloma (for UCC with inverted growth pattern) |
| IHC | GATA3 +ve, p63 +ve, CK5/6 +ve, CK34betaE12 +ve, PSA -ve |
| Site | urothelium - ureter, urinary bladder, proximal urethra (males), renal pelvis |
|
| |
| Syndromes | Lynch syndrome - esp. ureters |
|
| |
| Signs | hematuria |
| Prevalence | common |
| Prognosis | dependent on grade and stage |
| Treatment | dependent on grade and stage |
Urothelial carcinoma, also urothelial cell carcinoma, is a malignancy that arises from the urothelium. Urothelial carcinoma is abbreviated UC and urothelial cell carcinoma is abbreviated UCC.
This article deals with flat invasive urothelial carcinoma. The direct precursor is dealt with in urothelial carcinoma in situ.
Papillary urothelial carcinomas are dealt with in low-grade papillary urothelial carcinoma and high-grade papillary urothelial carcinoma.
See urine cytology for the cytopathology.
General
- These lesions lack papillae and are typical flat.
- Clinically, it may not be possible to differentiate renal pelvis urothelial carcinoma and renal cell carcinoma.
- May be a part of Lynch syndrome.
Prognosis:
- Women often have worse outcomes as they present with more advanced tumours.[1]
- Positive soft tissue margin.[2]
- Definition (radical cystectomy): tumour touching ink.
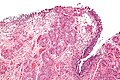
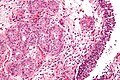
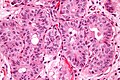
Microscopic
Features:
- Nuclear pleomorphism - key feature.
- Compare nuclei to one another.
- Increased N/C ratio.
- Lack of maturation to surface (important).
- Cells become dyscohesive.
- Mostly useless in my experience.
Invasion vs. in situ: Useful features - present in invasion:[3]
- Thin-walled vessels.
- Stromal reaction (hypercellularity).
- Retraction artefact around the tumour cell nests.
Note:
- The presence/absence of muscle should be commented on in biopsy specimens.
- Adipose tissue may be seen in the lamina propria; tumour adjacent to adipose tissue on a biopsy does not imply invasion deep to the muscularis propria.[4]
DDx:
- Pseudocarcinomatous urothelial hyperplasia.
- Urothelial carcinoma in situ.
- High-grade papillary urothelial carcinoma.
- Low-grade papillary urothelial carcinoma.
- Prostate carcinoma.
Staging
- T1 - lamina propria.
- Several subdivisions of T1 exist:
- T1a - superficial or in muscularis mucosae.
- T1b - beyond muscularis mucosae - into submucosa.
- Several subdivisions of T1 exist:
- T2 - muscularis propria.
Note:
- Approximately 25% of muscle invasive urothelial carcinoma on biopsy is a lower stage in the cystectomy specimen.[5]
- In approximately 15% of cases it is pT0 (no primary tumour identified).
Muscularis propria invasion
The presence or absence of muscularis propria invasion is a very important determination, as the clinical management changes between T1 and T2:
- T1: usually conservative treatment (local excision).
- T2: radical treatment (cystectomy or cystoprostatectomy).
A thin layer of discontinous muscularis mucuosae (MM) is present and, especially if hypertrophic, may be confused with muscuaris propria (MP).
Comparing MM and MP
A comparison between muscularis propria and muscularis mucosae - adapted from Paner et al.:[6]
| Feature | Muscularis mucosae | Muscularis propria |
|---|---|---|
| Outline/border | typically irregular (frayed edges) | usually regular (circumscribed) |
| Size of bundles ‡ | classically "small", often "large" (hypertrophic) | usually "large" |
| Isolated fibres | yes | no |
| Location in bladder | less common in trigone, dome very common | everywhere |
| Depth † | superficial, occ. deep | deep |
Notes:
- † The lamina propria thickness varies with location. It is thinnest in the trigone (0.5-1.6 mm) and thickest in the dome (1.0-3.1 mm).
- ‡ Small is defined as <4 muscle fibres; large >= 4 muscle fibres.
- The presence of hyperplastic bundles ranges from ~20% in the trigone to ~70% in the dome.
Rational assessment of muscularis propria invasion
To call muscularis propria invasion:
- Definite tumour must be between muscle.
- Muscle bundles must be thick.
- Multiple bundles must be adjacent to one another.
- Should not be superficial - surface epithelium if present should be distant.
Subtypes of urothelial carcinoma
There are numerous subtypes:[7]
- Squamous differentiation.
- Clear cell.
- Plasmacytoid.
- Micropapillary.
- Small nests (< ~10 cells/nest).
- Sarcomatoid.
- Many others...
Benign patterns - mnemonic Much GIN:
- Microcystic.
- Small tubular/glandular.
- Inverted.
- Nested.
Plasmacytoid urothelial cell carcinoma
Features:
- Abundant gray cytoplasm, eccentric nucleus.
Images:
Nested urothelial cell carcinoma
- AKA nested variant urothelial cell carcinoma.
Features:[8]
- High density of well-circumscribed nests.
- Mild-to-moderate nuclear atypia.
- +/-Foci of unequivocal conventional urothelial carcinoma.
- Focally solid or gland fusion.
- Moderate-to-severe nuclear atypia +/- abundant mitoses.
- +/-Extension into the muscularis propria.
DDx:
- von Brunn nests.
- Low grade papillary urothelial carcinoma with lamina propria invasion.
Images
www:
IHC
Recommended by ISUP consensus panel:[10]
- GATA3 +ve, CK20 +ve, p63 +ve, CK5/6, HMWCK (e.g. CK34betaE12) +ve.
Others:
- CK7 +ve.
- PSA -ve.
Notes:
- CK20 negative in over 50% of cases with metastases.[11]
Reactive changes versus UCIS
UCC versus other
UCC vs. prostate:
- UCC: GATA3 +ve, p63 +ve, CK20 +ve.
- Prostate: PSA +ve, PSAP +ve, CK7 -ve, CK20 -ve, p63 -ve, GATA3 -ve.
UCC vs. RCC:
- UCC: p63+.[12]
Staging
Staging - muscularis propria invasion versus muscularis mucosae invasion smoothelin stain:[13]
- Muscularis propria - usu. strong. †
- Muscularis mucosae - negative/weak. †
Note:
- † Overlap between the patterns is described,[14] this limits the utility of the stain.
Molecular
Not used for diagnosis.
Changes:
- 9p deletion -- site of CDKN2A[15] (AKA p16).
- 17p deletion -- site of PT53 (AKA p53).
Sign out
High grade UC
URINARY BLADDER LESION ("TUMOUR"), TRANSURETHRAL RESECTION URINARY BLADDER TUMOUR (TURBT):
- INVASIVE HIGH-GRADE PAPILLARY UROTHELIAL CARCINOMA WITH SQUAMOUS DIFFERENTIATION AT LEAST
INTO MUSCULARIS PROPRIA.
- LYMPHOVASCULAR INVASION PRESENT.
Nested variant
URINARY BLADDER LESION ("TUMOUR"), TRANSURETHRAL RESECTION OF BLADDER TUMOUR (TURBT):
- INVASIVE LOW-GRADE UROTHELIAL CARCINOMA, NESTED VARIANT.
- TUMOUR PRESENT AT EDGE OF TISSUE.
- NO MUSCULARIS PROPRIA IDENTIFIED.
UCC with some suspicion for muscularis propria invasion
URINARY BLADDER LESION ("TUMOUR"), DEEP, RE-RESECTION (TURBT):
- INVASIVE HIGH-GRADE UROTHELIAL CARCINOMA WITH SQUAMOUS DIFFERENTIATION AT
LEAST INTO THE LAMINA PROPRIA, SEE COMMENT.
- NO DEFINITE LYMPHOVASCULAR INVASION.
COMMENT:
Tumour is seen adjacent to smooth muscle fibres of intermediate thickness. This is
interpreted as thick muscularis mucosae. The tissue orientation is suboptimal.
Definite muscularis propria is not apparent. Levels were cut.
Tumour is abundant in the lamina propria.
Alternate comment
The sections shows thickened muscle bundles with frayed edges between the tumour cells. The muscle is thought to represent hypertrophic muscularis mucosae. The large extent of lamina propria invasion raises the possibility of a higher stage lesion that may not have been sampled.
See also
References
- ↑ Mitra, AP.; Skinner, EC.; Schuckman, AK.; Quinn, DI.; Dorff, TB.; Daneshmand, S. (Jan 2014). "Effect of gender on outcomes following radical cystectomy for urothelial carcinoma of the bladder: a critical analysis of 1,994 patients.". Urol Oncol 32 (1): 52.e1-9. doi:10.1016/j.urolonc.2013.08.007. PMID 24239476.
- ↑ Dotan, ZA.; Kavanagh, K.; Yossepowitch, O.; Kaag, M.; Olgac, S.; Donat, M.; Herr, HW. (Dec 2007). "Positive surgical margins in soft tissue following radical cystectomy for bladder cancer and cancer specific survival.". J Urol 178 (6): 2308-12; discussion 2313. doi:10.1016/j.juro.2007.08.023. PMID 17936804.
- ↑ Sternberg, SE. Histology for Pathologists. P.2047.
- ↑ Bochner, BH.; Nichols, PW.; Skinner, DG. (Mar 1995). "Overstaging of transitional cell carcinoma: clinical significance of lamina propria fat within the urinary bladder.". Urology 45 (3): 528-31. doi:10.1016/S0090-4295(99)80030-2. PMID 7879346.
- ↑ D'Souza, AM.; Pohar, KS.; Arif, T.; Geyer, S.; Zynger, DL. (Oct 2012). "Retrospective analysis of survival in muscle-invasive bladder cancer: impact of pT classification, node status, lymphovascular invasion, and neoadjuvant chemotherapy.". Virchows Arch 461 (4): 467-74. doi:10.1007/s00428-012-1249-4. PMID 22915241.
- ↑ Paner, GP.; Ro, JY.; Wojcik, EM.; Venkataraman, G.; Datta, MW.; Amin, MB. (Sep 2007). "Further characterization of the muscle layers and lamina propria of the urinary bladder by systematic histologic mapping: implications for pathologic staging of invasive urothelial carcinoma.". Am J Surg Pathol 31 (9): 1420-9. doi:10.1097/PAS.0b013e3180588283. PMID 17721199.
- ↑ URL: http://www.nature.com/modpathol/journal/v22/n2s/full/modpathol200926a.html. Accessed on: 19 August 2011.
- ↑ Talbert, ML.; Young, RH. (May 1989). "Carcinomas of the urinary bladder with deceptively benign-appearing foci. A report of three cases.". Am J Surg Pathol 13 (5): 374-81. PMID 2712189.
- ↑ Terada, T. (Oct 2011). "Nested variant of urothelial carcinoma of the urinary bladder.". Rare Tumors 3 (4): e42. doi:10.4081/rt.2011.e42. PMC 3282447. PMID 22355497. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3282447/.
- ↑ Amin MB, Epstein JI, Ulbright TM, et al. (August 2014). "Best practices recommendations in the application of immunohistochemistry in urologic pathology: report from the international society of urological pathology consensus conference". Am. J. Surg. Pathol. 38 (8): 1017–22. doi:10.1097/PAS.0000000000000254. PMID 25025364.
- ↑ Jiang, J.; Ulbright, TM.; Younger, C.; Sanchez, K.; Bostwick, DG.; Koch, MO.; Eble, JN.; Cheng, L. (Jul 2001). "Cytokeratin 7 and cytokeratin 20 in primary urinary bladder carcinoma and matched lymph node metastasis.". Arch Pathol Lab Med 125 (7): 921-3. doi:10.1043/0003-9985(2001)1250921:CACIPU2.0.CO;2. PMID 11419977.
- ↑ Langner, C.; Ratschek, M.; Tsybrovskyy, O.; Schips, L.; Zigeuner, R. (Aug 2003). "P63 immunoreactivity distinguishes upper urinary tract transitional-cell carcinoma and renal-cell carcinoma even in poorly differentiated tumors.". J Histochem Cytochem 51 (8): 1097-9. PMID 12871991.
- ↑ Paner, GP.; Shen, SS.; Lapetino, S.; Venkataraman, G.; Barkan, GA.; Quek, ML.; Ro, JY.; Amin, MB. (Jan 2009). "Diagnostic utility of antibody to smoothelin in the distinction of muscularis propria from muscularis mucosae of the urinary bladder: a potential ancillary tool in the pathologic staging of invasive urothelial carcinoma.". Am J Surg Pathol 33 (1): 91-8. doi:10.1097/PAS.0b013e3181804727. PMID 18936687.
- ↑ Miyamoto, H.; Sharma, RB.; Illei, PB.; Epstein, JI. (Mar 2010). "Pitfalls in the use of smoothelin to identify muscularis propria invasion by urothelial carcinoma.". Am J Surg Pathol 34 (3): 418-22. doi:10.1097/PAS.0b013e3181ce5066. PMID 20154589.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 600160