Difference between revisions of "High-grade prostatic intraepithelial neoplasia"
Jump to navigation
Jump to search
| (6 intermediate revisions by the same user not shown) | |||
| Line 5: | Line 5: | ||
| Caption = High-grade prostatic intraepithelial neoplasia. [[H&E stain]]. | | Caption = High-grade prostatic intraepithelial neoplasia. [[H&E stain]]. | ||
| Synonyms = prostatic intraepithelial neoplasia | | Synonyms = prostatic intraepithelial neoplasia | ||
| Micro = nuclear changes (hyperchromatic nuclei, nucleoli present, +/-increased NC ratio, mild-to-moderate nuclear enlargement), medium-to-large glands with the architecture of HGPIN (tufted, micropapillary | | Micro = nuclear changes (hyperchromatic nuclei, nucleoli present, +/-increased NC ratio, mild-to-moderate nuclear enlargement), medium-to-large glands with the architecture of HGPIN (tufted, micropapillary, flat) | ||
| Subtypes = | | Subtypes = | ||
| LMDDx = [[basal cell hyperplasia]], [[prostatic adenocarcinoma]], [[PIN-like prostatic ductal adenocarcinoma]], [[atypical small acinar proliferation]] (biopsy only) | | LMDDx = [[basal cell hyperplasia]], [[prostatic adenocarcinoma]], [[PIN-like prostatic ductal adenocarcinoma]], [[atypical small acinar proliferation]] (biopsy only), [[atypical intraductal proliferation]] | ||
| Stains = | | Stains = | ||
| IHC = AMACR +ve, basal cells present (p63 +ve, CK34betaE12 +ve) | | IHC = AMACR +ve, basal cells present (p63 +ve, CK34betaE12 +ve) | ||
| Line 80: | Line 80: | ||
**Nucleoli present - '''key (high power) feature'''. | **Nucleoli present - '''key (high power) feature'''. | ||
**Often increased NC ratio. | **Often increased NC ratio. | ||
**Nuclear enlargement. | **Nuclear enlargement - usually subtle/appreciated at high magnification only. | ||
*Tinctorial changes of the cytoplasm - usually amphophilic (red) or basophilic (blue). | |||
Notes: | Notes: | ||
*Nucleoli should be visible with the 20x objective. | *[[Nucleoli]] should be visible with the 20x objective. | ||
**If one uses the 40x objective... one over calls. | **If one uses the 40x objective... one over calls. | ||
*May need IHC for cancer versus HGPIN. | *May need IHC for cancer versus HGPIN. | ||
| Line 94: | Line 95: | ||
*[[Prostatic adenocarcinoma]] - glands with HGPIN have two or more distinct cells layers. | *[[Prostatic adenocarcinoma]] - glands with HGPIN have two or more distinct cells layers. | ||
**[[PIN-like prostatic ductal adenocarcinoma]] - glands crowded. | **[[PIN-like prostatic ductal adenocarcinoma]] - glands crowded. | ||
*Benign prostate - | *Benign prostate - HGPIN has nuclear changes. | ||
**Central zone typically has small nucleoli;<ref>{{Cite journal | last1 = Egevad | first1 = L. | title = Cytology of the central zone of the prostate. | journal = Diagn Cytopathol | volume = 28 | issue = 5 | pages = 239-44 | month = May | year = 2003 | doi = 10.1002/dc.10275 | PMID = 12722118 }}</ref> however, the glands are larger. | |||
*[[Atypical intraductal proliferation]] - a [[waffle diagnosis]] when criteria insufficient for [[intraductal carcinoma of the prostate]]. | |||
===HGPIN architecture=== | ===HGPIN architecture=== | ||
| Line 101: | Line 104: | ||
*Tufting - common. | *Tufting - common. | ||
*Micropapillary - common. | *Micropapillary - common. | ||
Notes: | |||
*The architectural pattern is '''not''' thought to have any prognostic significance; however, it may be useful for differentiating it from benign prostate. | *The architectural pattern is '''not''' thought to have any prognostic significance; however, it may be useful for differentiating it from benign prostate. | ||
*"Cribriform HGPIN" previously existed; it is now classified as [[atypical intraductal proliferation]].<ref name=pmid35758185>{{cite journal |authors=Kench JG, Amin MB, Berney DM, Compérat EM, Cree IA, Gill AJ, Hartmann A, Menon S, Moch H, Netto GJ, Raspollini MR, Rubin MA, Tan PH, Tsuzuki T, Turjalic S, van der Kwast TH, Zhou M, Srigley JR |title=WHO Classification of Tumours fifth edition: evolving issues in the classification, diagnosis, and prognostication of prostate cancer |journal=Histopathology |volume=81 |issue=4 |pages=447–458 |date=October 2022 |pmid=35758185 |pmc=9542779 |doi=10.1111/his.14711 |url=}}</ref> | |||
===Images=== | ===Images=== | ||
Latest revision as of 17:21, 3 April 2024
| High-grade prostatic intraepithelial neoplasia | |
|---|---|
| Diagnosis in short | |
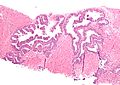
 High-grade prostatic intraepithelial neoplasia. H&E stain. | |
|
| |
| Synonyms | prostatic intraepithelial neoplasia |
|
| |
| LM | nuclear changes (hyperchromatic nuclei, nucleoli present, +/-increased NC ratio, mild-to-moderate nuclear enlargement), medium-to-large glands with the architecture of HGPIN (tufted, micropapillary, flat) |
| LM DDx | basal cell hyperplasia, prostatic adenocarcinoma, PIN-like prostatic ductal adenocarcinoma, atypical small acinar proliferation (biopsy only), atypical intraductal proliferation |
| IHC | AMACR +ve, basal cells present (p63 +ve, CK34betaE12 +ve) |
| Gross | not evident |
| Site | prostate gland |
|
| |
| Associated Dx | prostate adenocarcinoma |
| Signs | none |
| Symptoms | none |
| Prevalence | common |
| Blood work | +/-PSA elevated |
| Radiology | not identifiable |
| Prognosis | benign |
| Clin. DDx | prostate carcinoma |
| Treatment | follow-up +/-re-biopsy |
High-grade prostatic intraepithelial neoplasia, abbreviated as HGPIN, is considered the precursor for prostate carcinoma.
It may be referred to as prostatic intraepithelial neoplasia, abbreviated PIN.
General
- Thought to be a precursor lesion for prostate adenocarcinoma.
- Incidence ~5-8% on core biopsy.[1]
- Multifocal HGPIN considered a risk for prostate cancer on re-biopsy.[2][3]
- A small focus of HGPIN does not appear to be associated with an increased risk for prostate cancer on re-biopsy at one year if the initial biopsy had 8 or more cores.[4]
- Interrater variability is moderate (kappa ~ 0.45) for benign versus HGPIN.[5]
Low-grade prostatic intraepithelial neoplasia:
- Should not be reported.[1]
- Believed to be irrelevant biologically/clinically.
- PIN not otherwise specified refers to HGPIN.
- Low-grade PIN has the architecture of HGPIN but lacks the nuclear atypia.
HGPIN and cancer on follow-up biopsy
Prostate cancer on follow-up biopsy by number of HGPIN sites from Merrimen et al.:[3]
| Number of cores with HGPIN |
Odds ratio of cancer on follow-up (95% CI) |
|---|---|
| 0 | 1.00 (reference) |
| 1 | 1.02 (0.73-1.40) |
| 2 | 1.55 (1.08-2.21) |
| 3 | 1.99 (1.16-3.40) |
| 4 | 2.66 (1.10-6.40) |
Gross
- Not evident on gross.
Microscopic
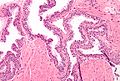
- Medium to large glands with architectural changes - see HGPIN architecture below.
- Described as "epithelial hyperplasia".
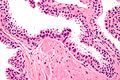
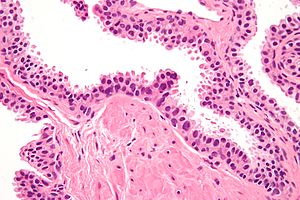
- Diagnosed on basis of nuclear changes.
- Hyperchromatic nuclei - key (low power) feature.
- Nucleoli present - key (high power) feature.
- Often increased NC ratio.
- Nuclear enlargement - usually subtle/appreciated at high magnification only.
- Tinctorial changes of the cytoplasm - usually amphophilic (red) or basophilic (blue).
Notes:
- Nucleoli should be visible with the 20x objective.
- If one uses the 40x objective... one over calls.
- May need IHC for cancer versus HGPIN.
- Nucleoli should be present in >= 10% of cells in a gland to call it HGPIN.[8]
- This criterium is not required by all pathologists.
DDx:
- Basal cell hyperplasia of the prostate.
- Intraductal carcinoma of the prostate.
- Prostatic adenocarcinoma - glands with HGPIN have two or more distinct cells layers.
- PIN-like prostatic ductal adenocarcinoma - glands crowded.
- Benign prostate - HGPIN has nuclear changes.
- Central zone typically has small nucleoli;[9] however, the glands are larger.
- Atypical intraductal proliferation - a waffle diagnosis when criteria insufficient for intraductal carcinoma of the prostate.
HGPIN architecture
There are several forms:[10][11]
- Flat - uncommon.
- Tufting - common.
- Micropapillary - common.
Notes:
- The architectural pattern is not thought to have any prognostic significance; however, it may be useful for differentiating it from benign prostate.
- "Cribriform HGPIN" previously existed; it is now classified as atypical intraductal proliferation.[12]
Images
IHC
- HGPIN: AMACR +ve, p63 +ve, HMWCK +ve.
- Cancer: AMACR +ve, p63 -ve, HMWCK -ve.
- Normal: AMACR -ve‡, p63 +ve, HMWCK +ve.
Note:
- ‡ May be positive in normal prostate.[13]
Sign out
A. PROSTATE, RIGHT LATERAL SUPERIOR, BIOPSY: - HIGH-GRADE PROSTATIC INTRAEPITHELIAL NEOPLASIA; - NEGATIVE FOR MALIGNANCY.
Mostly lower case
A. Left Apex: - Focal high grade prostatic intraepithelial neoplasia, chronic inflammation B. Left Middle Zone: - Benign prostatic tissue C. Left Base: - Benign prostatic tissue D. Right Apex: - Benign prostatic tissue E. Right Middle Zone: - Benign prostatic tissue F. Right Base: - Benign prostatic tissue
Colour seen in tissue cores at sign-out: green
Comment for many cores with HGPIN
If there is (isolated) HGPIN in more than 3 or 4 cores:
COMMENT: As high-grade prostatic intraepithelial neoplasia is found in multiple cores, close follow-up is suggested, with a re-biopsy when indicated.
TURP
PROSTATE TISSUE, TRANSURETHRAL RESECTION OF THE PROSTATE (TURP): - HIGH-GRADE PROSTATIC INTRAEPITHELIAL NEOPLASIA (HGPIN), FOCAL. - ACUTE AND CHRONIC INFLAMMATION. - UROTHELIUM WITH MILD INFLAMMATION. - NEGATIVE FOR MALIGNANCY.
See also
References
- ↑ 1.0 1.1 Epstein, JI.; Herawi, M. (Mar 2006). "Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care.". J Urol 175 (3 Pt 1): 820-34. doi:10.1016/S0022-5347(05)00337-X. PMID 16469560.
- ↑ Srigley, JR.; Merrimen, JL.; Jones, G.; Jamal, M. (Dec 2010). "Multifocal high-grade prostatic intraepithelial neoplasia is still a significant risk factor for adenocarcinoma.". Can Urol Assoc J 4 (6): 434. PMID 21191509.
- ↑ 3.0 3.1 Merrimen, JL.; Jones, G.; Walker, D.; Leung, CS.; Kapusta, LR.; Srigley, JR. (Aug 2009). "Multifocal high grade prostatic intraepithelial neoplasia is a significant risk factor for prostatic adenocarcinoma.". J Urol 182 (2): 485-90; discussion 490. doi:10.1016/j.juro.2009.04.016. PMID 19524976.
- ↑ Herawi, M.; Kahane, H.; Cavallo, C.; Epstein, JI. (Jan 2006). "Risk of prostate cancer on first re-biopsy within 1 year following a diagnosis of high grade prostatic intraepithelial neoplasia is related to the number of cores sampled.". J Urol 175 (1): 121-4. doi:10.1016/S0022-5347(05)00064-9. PMID 16406886.
- ↑ Allam, CK.; Bostwick, DG.; Hayes, JA.; Upton, MP.; Wade, GG.; Domanowski, GF.; Klein, MA.; Boling, EA. et al. (Jul 1996). "Interobserver variability in the diagnosis of high-grade prostatic intraepithelial neoplasia and adenocarcinoma.". Mod Pathol 9 (7): 742-51. PMID 8832557.
- ↑ Amin, Mahul B. (2010). Diagnostic Pathology: Genitourinary (1st ed.). Amirsys. pp. 3-56. ISBN 978-1931884280.
- ↑ Chin, AI.; Dave, DS.; Rajfer, J. (2007). "Is repeat biopsy for isolated high-grade prostatic intraepithelial neoplasia necessary?". Rev Urol 9 (3): 124-31. PMC 2002502. PMID 17934569. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2002502/.
- ↑ Amin, Mahul B. (2010). Diagnostic Pathology: Genitourinary (1st ed.). Amirsys. pp. 3-55. ISBN 978-1931884280.
- ↑ Egevad, L. (May 2003). "Cytology of the central zone of the prostate.". Diagn Cytopathol 28 (5): 239-44. doi:10.1002/dc.10275. PMID 12722118.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 380. ISBN 978-0781765275.
- ↑ Bostwick, DG.; Qian, J. (Mar 2004). "High-grade prostatic intraepithelial neoplasia.". Mod Pathol 17 (3): 360-79. doi:10.1038/modpathol.3800053. PMID 14739906. http://www.nature.com/modpathol/journal/v17/n3/pdf/3800053a.pdf.
- ↑ Kench JG, Amin MB, Berney DM, Compérat EM, Cree IA, Gill AJ, Hartmann A, Menon S, Moch H, Netto GJ, Raspollini MR, Rubin MA, Tan PH, Tsuzuki T, Turjalic S, van der Kwast TH, Zhou M, Srigley JR (October 2022). "WHO Classification of Tumours fifth edition: evolving issues in the classification, diagnosis, and prognostication of prostate cancer". Histopathology 81 (4): 447–458. doi:10.1111/his.14711. PMC 9542779. PMID 35758185. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9542779/.
- ↑ Ananthanarayanan, V.; Deaton, RJ.; Yang, XJ.; Pins, MR.; Gann, PH. (Jun 2005). "Alpha-methylacyl-CoA racemase (AMACR) expression in normal prostatic glands and high-grade prostatic intraepithelial neoplasia (HGPIN): association with diagnosis of prostate cancer.". Prostate 63 (4): 341-6. doi:10.1002/pros.20196. PMID 15602744.