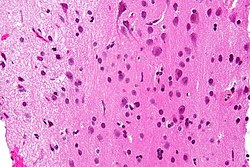
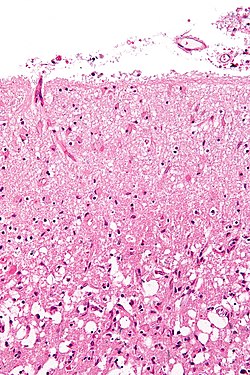
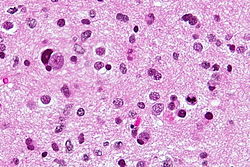
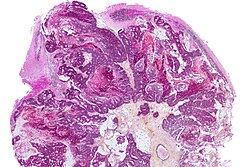
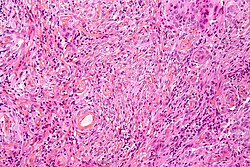
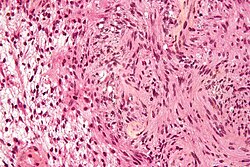
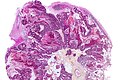
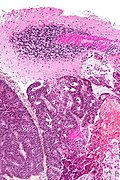
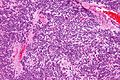
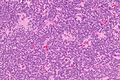
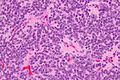
Neuropathology tumours
The article covers tumours in neuropathology. Tumours are a large part of neuropathology. Cytopathology of CNS tumours is dealt with in the article CNS cytopathology.
There are separate articles for peripheral nerve sheath tumours and pituitary/peri-pituitary lesions.
Brain tumours - overview
Adult
Four most common types of brain tumours:[1]
- Metastatic brain tumours (barely edges out primary tumours)
- Lung (most common).
- Breast.
- Melanoma.
- Renal cell carcinoma (RCC).
- Glioblastoma (previously known as glioblastoma multiforme).
- Anaplastic astrocytoma.
- Meningioma.
Children
- Astrocytoma.
- Medulloblastoma.
- Ependymoma.
Location (most common)
Certain tumours like to hang-out at certain places:[2]
- Cerebrum:
- Cortical based - oligodendroglioma.
- Grey-white junction - metastases.
- White matter - astrocytoma, glioblastoma.
- Periventricular - CNS lymphoma.
- Cystic - ganglioglioma, pilocytic astrocytoma, pleomorphic xanthoastrocytoma.
- Cerebellum:
- Midline/central - medulloblastoma.
- Cystic lesion - pilocytic astrocytoma (younger individual), hemangioblastoma (older individual).
- Solid lesion (older individual) - metastasis.
- Spinal cord:
- Ependymoma, glioblastoma.
- Filum terminale - myxopapillary ependymoma, paraganglioma.
Filum terminale
- Filum terminale = bottom end of the spinal cord - has a limited differential.
DDx:[3]
Cerebellopontine angle
- Abbreviated CP angle.
DDx:[4]
- Schwannoma.
- Meningioma.
- Dermoid cyst/epidermoid cyst.
- Ependymoma.
- Choroid plexus papilloma.
Cystic tumours
DDx:[5]
- Pilocytic astrocytoma.
- Pleomorphic xanthoastrocytoma.
- Ganglioglioma.
- Hemangioblastoma.
- Craniopharyngioma.[6]
Primary versus secondary
- AKA (primary) brain tumour versus metastatic cancer.
Primary
Glial tumours:
- Cytoplasmic processes - key feature.
- Best seen at highest magnification - usu. ~1 micrometer.
- Processes may branch.
- Ill-defined border/blend with the surrounding brain.
- Large (lymphoid) cells, ergo usu. not a difficult diagnosis.
- ~2x size of resting lymphocyte, nucleoli.
- Lesion predominantly perivascular.
Secondary
Carcinomas:
- Well-demarcated border between brain and lesion - key feature.
- No cytoplasmic processes.
- Usu. have nuclear atypia of malignancy.
- Nuclei often ~3-4x the size of a RBC.
- +/-Glandular arrangement.
- +/-Nucleoli.
Common neuropathology tumours in a table
| Type | Key feature(s) | Imaging | History | Notes | IHC | Images |
| Normal tissue | cells regularly spaced, no nuc. atypia | small lesion? / deep lesion? | variable | missed lesion? | nil | |
| Reactive astrocytes | astrocytes with well-demarcated eosinophilic cytoplasm, regular spacing, no nuc. atypia | small lesion? / deep lesion? | variable | missed lesion / close to a lesion; non-specific pathologic process - need more tissue | nil | |
| Astrocytoma (grade II or worse) | glial processes (esp. on smear), nuclear atypia (size var. ~3x, irreg. nuc. membrane, hyperchromasia), no Rosenthal fibres in the core of the lesion † | often enhancing (suggests high grade), usu. supratentorial, usu. white matter | usu. old, occ. young | very common, esp. glioblastoma | IDH-1+/-, GFAP+ | |
| Metastasis | sharp interface with brain, often glandular, +/-nucleoli, no glial processes | often cerebellular, well-circumscribed | usu. old | often suspected to have metastatic disease | TTF-1, CK7, CK20, BRST-2 | |
| Meningioma | whorls, psammomatous calcs, nuclear inclusions | extra-axial + intradural | old or young | may be diagnosed on smear, DDx: schwannoma, choroid plexus | EMA, PR, Ki-67 | |
| Schwannoma | cellular areas (Antoni A), paucicelluar areas (Antoni B), palisading of nuclei (Verocay bodies) | extra-axial + intradural | old or young | need frozen section to Dx, DDx: meningioma | S100 |
† Rosenthal fibres at the periphery of a lesion are a non-specific finding seen in chronic processes.
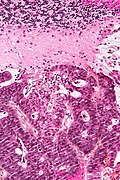
Metastatic brain tumours
General
- Most common brain tumour in adults.
- Common metastases (in order): lung, breast, kidney, gastrointestinal, melanoma.Greenwald, J.; Heng, M. (2007). Toronto Notes for Medical Students 2007 (2007 ed.). The Toronto Notes Inc. for Medical Students Inc.. pp. NS9. ISBN 978-0968592878.
- Percentage of previously diagnosed cancers with brain metastases - by primary site: lung cancer 19.9%, melanoma 6.9%, breast cancer 5.1%, renal cancer 6.5%, colorectal cancer 1.8%.[7]
- Lung followed by kidney is the order in a smaller series.[8]
Microscopic
Appearance varies by subtype.
Features of metastatic carcinoma:
- Tubule formation/glands.
- Usually well-circumscribed/sharply demarcated from surrounding tissue.
- Usually nuclear atypia including:
- Nuclear hyperchromasia.
- Variation of nuclear size.
- Variation of nuclear shape.
- Mitoses - common.
DDx:
- Primary brain tumour - see primary brain tumour versus secondary brain tumour.
Images
CRC metastasis to cerebellum - very low mag. (WC)
IHC
- Carcinoma: pankeratin +ve.
- Lung adenocarcinoma and SCLC: TTF-1 +ve, CK7 +ve, CK20 -ve.
- Breast carcinoma: CK7 +ve, ER +ve, PR +ve, BRST2 +ve/-ve.
- Colorectal carcinoma: CK7 -ve, CK20 +ve, CDX2 +ve, TTF-1 -ve.
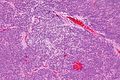
Infiltrative astrocytomas
Overview
- Low-grade (diffuse) astrocytomas (Grade II).
- Anaplastic astrocytomas (Grade III).
- Glioblastoma (Grade IV).
Notes:
- Non-infiltrative gliomas:
- Pilocytic astrocytoma (WHO Grade I).
- Dysembryoplastic neuroepithelial tumour (DNT), (WHO Grade I).
Microscopic
- Glial processes - key feature.
- Thin stringy cytoplasmic processes - best seen at high power in less cellular areas.
- No Rosenthal fibres within the tumour itself.
Images:
- Endothelial proliferation in a GBM (ouhsc.edu).
- Endothelial proliferation (ouhse.edu).
- Gemistocytic astrocytoma - several images (upmc.edu).
Notes:
- Glial vs. non-glial tumours:
- Glial: "blends into brain"/gradual transition to non-tumour brain.
- Non-glial: no glial processes.
- Rosenthal fibres within the tumour... make it into a pilocytic astrocytoma.
- Rosenthal fibres may be seen around a (very) slow growing tumour and represent a reactive process.
- Inflammatory cells and macrophages should prompt consideration of an alternate diagnosis (e.g. cerebral infarct, multiple sclerosis) - esp. if this is a primary lesion.[11]
Grading
Nuclear pleomorphism present:
- At least grade II (diffuse astrocytoma).
Mitotic figures present:
- At least grade III (anaplastic astrocytoma).
Microvascular proliferation or necrosis with pseudopalisading tumour cells:
- Grade IV (glioblastoma AKA glioblastoma multiforme).
Notes:
- Pseudopalisading tumour cells = high tumour cell density adjacent to regions of necrosis; palisade = a fence of pales forming a defense barrier or fortification.
Images:
- Glioblastoma:
- Anaplastic astrocytoma:
Table of common gliomas - grading
Histomorphologic comparison of common gliomas:
| Entity | Rosenthal fibres / EGBs |
Nuclear atypia | Mitoses | Necrosis or MVP | Infiltrative | Image |
| Pilocytic astrocytoma | yes | usu. no | usu. no | usu. no | no | [1] |
| Low-grade astrocytoma | no | yes | no | no | yes | image? |
| Anaplastic astrocytoma | no | yes | yes | no | yes | [2] |
| Glioblastoma | no | yes | yes | yes | yes | [3] |
Notes:
- MVP = microvascular proliferation.
- EGBs = eosinophilic granular bodies.
IHC
- GFAP - should stain cytoplasm of tumour cells and the perikaryon (nuclear membrane).
- Ki-67 - usu. high >20% of cells.
- p53 - often +ve.
- IDH1 (isocitrate dehydrogenase 1).
- +ve in tumours that arose from low-grade gliomas.[12]
- Image: IDH1 +ve in glioblastoma (WP).
- +ve in tumours that arose from low-grade gliomas.[12]
Notes:
- IDH1 and IDH2 mutations - better survival.[13]
Pilocytic astrocytoma
Pleomorphic xanthoastrocytoma
- Abbreviated PXA.
General
Features:
- Classically in the temporal lobe in children and young adults.
- Associated with seizures.
- Moderately aggressive (WHO Grade II).[14]
Gross
- Temporal lobe - classic.
- Usually assoc. with the leptomeninges,[14] i.e. superficial.
Microscopic
Features:[15]
- Marked nuclear atypia.
- Eosinophilic granular bodies - very common.[14]
- Inflammation (chronic).
Notes:
- No mitoses.
- No necrosis.
Images:
- Pleomorphic xanthoastrocytoma - several images (upmc.edu).
- Pleomorphic xanthoastrocytoma with anaplasia - another case - several images (upmc.edu).
- Pleomorphic xanthoastrocytoma with anaplasia - case 3 - several images (upmc.edu).
- Cerebellar pleomorphic xanthoastrocytoma - case 4 - several image (upmc.edu).
Stains
- Reticulin stain - intercellular, prominent.[16]
Image:
IHC
- GFAP +ve.
- CD68 +ve.
Dysembryoplastic neuroepithelial tumour
- Abbreviated DNT.
General
- Common tumour cause of drug resistant epilepsy.[17]
- Paediatric population.
Gross/radiology
- Temporal lobe.
- Variable architecture:[18] cystic, solitary nodular, multinodular.
Microscopic
Features:[18]
- Cells similar to oligodendrocytes:
- Large central nuclei with indentations.
- Multiple small nucleoli (common).
- Clear cytoplasm.
DDx:
- Oligodendroglioma.
- These have rounder, smaller nuclei with occasional nucleoli.[18]
Images:
Subependymal giant cell astrocytoma
- Abbreviated SEGA.
General
- Associated with tuberous sclerosis complex (TSC).[19]
- WHO Grade I.
Gross/radiology
- Well-demarcated.
Microscopic
- Giant cells with nuclear atypia ("bizarre cells").
- Vesicular nuclei.
- Nuclear pseudoinclusions.[22]
- Glassy eosinophilic cytoplasm.
- Abundant mast cells.[22]
Images:
IHC
Features:[21]
- GFAP +ve. (???)
- Vimentin +ve. (???)
- S100 +ve. (???)
Pilomyxoid astrocytoma
General
Features:[23]
- A variant of pilocytic astrocytoma.
- Some have suggested it is a unique entity.[24]
- Childhood or adolescence.
Gross
Features:[23]
- Classically - hypothalamic location/suprasellar location; may involve the sella turcica.[25]
- Solid.
- Well-circumscribed.
Microscopic
Features:[23]
- Consists of small round/ovoid bland cells in a myxoid stroma.
- Hair-like fibres ~ 1 micrometer.
- Often difficult to appreciate on standard (H&E) histologic sections.
- Usually angiocentric (surround blood vessel) - key feature.
Notes:[23]
- Rosenthal fibres are absent - key negative.
- Monophasic (unlike classical pilocytic astrocytomas) - key negative.
- May rarely have eosinophilic granular bodies.
Grading
- WHO Grade II by definition.[23]
Atypical teratoid/rhabdoid tumour
- See also: Extrarenal malignant rhabdoid tumour.
- Commonly abbreviated AT/RT.
- May be written atypical teratoid rhabdoid tumour, i.e. without the forward slash, or atypical teratoid-rhabdoid tumour (AT-RT).
Oligodendroglioma
Oligoastrocytoma
General
- Mixed tumour.
Microscopic
Features:
- Astrocytoma-like and oligodendroglioma-like:
- Oligodendroglioma-like cells = round nucleus, peri-nuclear clearing.
- Astrocytoma-like cells = non-ovoid/elongated nucleus.
DDx:
- Anaplastic astrocytoma.
- Oligodendroglioma. (???)
IHC
- Oligodendroglioma-like cells: MAP-2 +ve (cytoplasm).
- Astrocytoma-like cells: GFAP +ve (cytoplasm, nuclear membrane).
Others:
- Ki-67 ~10%. (???)
- p53 - focally +ve. (???)
- IDH-1 -ve. (???)
Meningioma
General
- Very common.
- May be part of a syndrome.
Microscopic
Features (memory device WCN):
- Whorled appearance - key feature.
- Calcification, psammomatous.
- Nuclear pseudoinclusions - focal nuclear clearing with a sharp interface to unremarkable chromatin.
Grading: see meningioma.
Peripheral nerve sheath tumours
A classification:[26]
- Benign:
- Malignant:
Schwannoma
General
- Tumour of tissue surrounding a nerve.
- Axons adjacent to the tumour are normal... but may be compressed.
Microscopic
Features:[26]
- Antoni A:
- Cellular.
- 'Fibrillary, polar, elongated'.
- Antoni B:
- Pauci-cellular.
- Loose microcystic tissue.
- Verocay bodies - paucinuclear area surrounded by palisaded nuclei.
- In the GI tract: classically have a peripheral lymphoid cuff.[27]
Images:
Notes:
- Several subtypes exist.
Neurofibroma
General
- May be a part of neurofibromatosis 1.
- Composed of Schwann cells, axons, fibrous material.[26]
Microscopic
Features:
- Spindle cells lesion.
- See Neurofibroma article for details.
Image:
Ganglioneuroma
- Not to be confused with ganglioglioma.
General
- May be retroperitoneal.
- Occasionally found in the GI tract - may form colonic polyp.
- Multiple ganglioneuromas may be due to multiple endocrine neoplasia IIb.
Classification:
- In a grouping known as neuroblastic tumours which includes:[29]
- Ganglioneuroma (benign).
- Ganglioneuroblastoma (intermediate).
- Neuroblastoma (aggressive).
Gross
- Solid.
- White.
- Firm.
- Well-circumscribed.
- May be nodular.
Images:
Microscopic
Features:
- Ganglion cells - key feature.
- Large cells with large nucleus.
- Prominent nucleolus.
- Large cells with large nucleus.
- Disordered fibrinous-like material.
- Eosinophilic granular bodies.[30]
Images:
- Ganglioneuroma (WC).
- Ganglioneuroma (webpathology.com).
- Ganglioneuroma (webpathology.com).
- Normal ganglion - high mag. (WC) .
See: adrenal ganglioneuroma, colonic ganglioneuroma.
IHC
Features:[31]
- Spindle cells: S-100 +ve.
- Ganglion cells: NSE, synaptophysin, NF.
Ependymoma
General
- Called the forgotten glial tumour.
Epidemiology:[32]
- Usual site:
- Adults: usu. spinal cord.
- Children: usu. posterior fossa.
- May be assoc. with neurofibromatosis 2.
Comes in two main flavours:
- Ependymoma (not otherwise specified).
- Myxopapillary ependymoma.
- Classically at filum terminale.
Other flavours:[33]
- Papillary ependymoma.
- Clear cell ependymoma.
Microscopic
Classic ependymoma
Features:
- Cells have a "tadpole-like" morphology.
- May also be described as ice cream cone-shaped.[34]
- Rosettes = circular nuclear free zones/cells arranged in a pseudoglandular fashion; comes in two flavours in ependymoma:
- Perivascular pseudorosettes = (tumour) cells arranged around a blood vessel; nuclei of cells distant from the blood vessel, i.e. rim of cytoplasm (from tumour cells) surround blood vessel (nucleus-free zone); more common than ependymal rosette... but less specific.
- Ependymal rosette (AKA true ependymal rosette) = rosette has an empty space at the centre - key feature.
- Nuclear features monotonous, i.e. "boring".[35]
- There is little variation in size, shape and staining.
DDx (classic ependymoma):
- Subependymoma.
- Glioblastoma (GBM).
- Invasive border = GBM; circumscribed border of lesion = ependymoma.
Images:
- www:
- WC:
Myxopapillary ependymoma
Features:
- Perivascular pseudorosettes:
- Myxoid material surround blood vessels.
- Myxoid material surrounded by tumour cells.
- Myxoid material surround blood vessels.
Images:
- Myxopapillary ependymoma - high mag. (WC).
- Myxopapillary ependymoma (bmj.com) - part of careers.bmj.com article on paediatric pathology.
- Myxopapillary ependymoma - cytology (WC).
- Myxopapillary ependymoma - several images (upmc.edu).
Grading
Easy:
- Subependymoma = WHO grade I.
- Myxopapillary ependymoma = WHO grade I.
Not-so-easy:
- Classic ependymoma = WHO grade II.
- Anaplastic ependymoma = WHO grade III.
Grade II vs. Grade III:
- Cellular density.
- Mitoses.
- Necrosis.
- Microvascular proliferation.
Notes:
- Many tumours fall between grade II and grade III. These are called "indeterminate" by many.
IHC
- Reticulin.
- GFAP.
- MIB1.
Subependymoma
Choroid plexus papilloma
Choroid plexus carcinoma
General
- Usually pediatric population.
- Malignant counterpart of choroid plexus papilloma.[36]
- Poor prognosis - WHO grade III.[37]
- Classically posterior fossa.
- Intraventricular mass.
Microscopic
Features:[36]
- Choroid plexus epithelium with nuclear pleomorphism & high NC ratio.
- Mitoses.
- Necrosis.
- +/-Brain invasion.
DDx:
- Choroid plexus papilloma.
- Atypical plexus papilloma - has features intermediate between choroid plexus papilloma and choroid plexus carcinoma.[37]
- Atypical teratoid/rhabdoid tumour.
Images:
IHC
Features:[37]
- Cytokeratins +ve.
- EMA usu. -ve.
- GFAP -ve (~20% +ve).
- Ki-67 high.
- Useful to diff. from benign counterpart.
- INI1 +ve.
Chordoma
Hemangioblastoma
Medulloblastoma
- Tumour of cerebellum - key feature.
- Morphologically identical supratentorial tumours are called primitive neuroectodermal tumour (PNET).
Primitive neuroectodermal tumour
- AKA primitive neuroepithelial tumour. (???)
General
- Abbreviated PNET.
- Should not be confused with peripheral primitive neuroectodermal tumour (abbreviated pPNET[38]), AKA Ewing sarcoma.
Microscopic
Features:
DDx:
Images:
- Primitive neuroectodermal tumour - several images (upmc.edu).
- GBM with PNET component - several images (upmc.edu).
Embryonal tumour with abundant neuropil and true rosettes
- Abbreviated ETANTR.
General
- Super rare.
- Reported only in children <4 years old.[40]
Microscopic
Features:[41]
- Small round blue cell tumour.
- True rosettes = flower-like cluster of cells that surrounds a space.[40]
- Fibrillary neuropil.
- Meshwork of fibers.
DDx:
Images:
CNS lymphoma
Classification:
- Primary CNS lymphoma.
- Non-primary CNS lymphoma - see lymphoma article.
General - primary CNS
- Classically periventicular distribution.
- Usually large B cell; can be considered a type of diffuse large B cell lymphoma (DLBCL).
- Prognosis of CNS (DLBCL) lymphomas worse than nodal (non-CNS) DLBCL.[42]
Microscopic
Features:
- Large cell lymphoma.
- Size = 2x diameter normal lymphocyte.
- Nucleolus - common.
- Perivascular clustering.
Images
www:
IHC
Can be subclassified in GCB (germinal centre B-cell-like) and non-GCB by CD10, Bcl-6, MUM1/IRF-4, and Bcl-2.[42]
Common pattern:
- CD20 +ve - key stain.
- CD3 -ve.
- Ki-67 ~40%.
- Bcl-6 +ve.
- Bcl-1 -ve.
Neurocytoma
General
- Rare.
Microscopic
Features:[43]
- Pineocytomatous/neurocytic rosette = irregular rosette with a large meshwork of fibers (neuropil) at the centre.[40]
- Similar to Homer-Wright rosette.
- Perinuclear clearing.
- Well-defined cell borders.
DDx:
- Oligodendroglioma - do not have the characteristic rosettes.
- Ganglioglioma.
- Ependymoma.
Images:
- Neurocytoma (ouhsc.edu).
- Neurocytoma - several images (upmc.edu).
- Neurocytoma - cerebellar - several images (upmc.edu).
IHC
- Synaptophysin +ve.
- Most glial tumour -ve.[44]
Central neurocytoma
- Abbreviated CNC.
General
Gross/radiology
Microscopic
Features:[48]
- Perivascular pseudorosette = circular/flower-like arrangement of cells with blood vessel at the centre.[40]
- Islands of neuropil.
- Polygonal cells with a perinuclear halo.
DDx:
DDx of perivascular pseudorosette:
- Ependymoma.
- Medulloblastoma, PNET.
- Glioblastomas.
Images
www:
- Central neurocytoma - several images (ouhsc.edu).
- Central neurocytoma (frontalcortex.com).
- Central neurocytoma - crappy images (upmc.edu).
IHC
- MIB1 - high may predict re-occurance.[49]
Ganglioglioma
- Not to be confused with ganglioneuroma.
General
Microscopic
Features:
- Atypical neurons.
- Atypical glia.
Images:
Lhermitte-Duclos disease
- Abbreviated LDD.
- AKA dysplastic cerebellar gangliocytoma.[51]
- AKA dysplastic gangliocytoma of the cerebellum.
Ganglioneuroblastoma
General
- Uncommon.
- Part of the neuroblastic tumours group which includes:[29]
- Ganglioneuroma (benign).
- Ganglioneuroblastoma (intermediate).
- Neuroblastoma (aggressive).
Microscopic
Features:
- Ganglion-like cells with a prominent nucleolus.
- Small undifferentiated cells with scant cytoplasm.
Images:
IHC
- NSE +ve -- small cells.
Lesions of the sella turcica
Lesions of the sella turcica, the pituitary gland environs, is a topic for it self. The differential diagnosis for lesions in this area includes:
- Pituitary adenoma.
- Craniopharyngioma.
- Rathke cleft cyst.
- Germ cell tumour.
- Meningioma.
- Pilomyxoid astrocytoma - in children.
See also
References
- ↑ http://neurosurgery.mgh.harvard.edu/abta/primer.htm
- ↑ URL: http://www.msdlatinamerica.com/ebooks/DiagnosticNeuropathologySmears/files/4ce563fb7e8e48fc9ed8b42e296a7747.gif and http://www.msdlatinamerica.com/ebooks/DiagnosticNeuropathologySmears/sid117213.html. Accessed on: 2 November 2010.
- ↑ JLK. 31 May 2010.
- ↑ R. Kiehl. 8 November 2010.
- ↑ URL: http://path.upmc.edu/cases/case320/dx.html. Accessed on: 14 January 2012.
- ↑ URL: http://www.pathologyoutlines.com/Cnstumor.html#cystsgeneral. Accessed on: 14 January 2012.
- ↑ Barnholtz-Sloan, JS.; Sloan, AE.; Davis, FG.; Vigneau, FD.; Lai, P.; Sawaya, RE. (Jul 2004). "Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System.". J Clin Oncol 22 (14): 2865-72. doi:10.1200/JCO.2004.12.149. PMID 15254054.
- ↑ Schouten, LJ.; Rutten, J.; Huveneers, HA.; Twijnstra, A. (May 2002). "Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma.". Cancer 94 (10): 2698-705. PMID 12173339.
- ↑ Rong Y, Durden DL, Van Meir EG, Brat DJ (June 2006). "'Pseudopalisading' necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis". J. Neuropathol. Exp. Neurol. 65 (6): 529–39. PMID 16783163.
- ↑ http://dictionary.reference.com/browse/palisading
- ↑ URL: http://path.upmc.edu/cases/case79/dx.html. Accessed on: 2 January 2012.
- ↑ Yan H, Parsons DW, Jin G, et al. (February 2009). "IDH1 and IDH2 mutations in gliomas". N. Engl. J. Med. 360 (8): 765–73. doi:10.1056/NEJMoa0808710. PMC 2820383. PMID 19228619. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2820383/.
- ↑ Houillier C, Wang X, Kaloshi G, et al. (October 2010). "IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas". Neurology 75 (17): 1560–6. doi:10.1212/WNL.0b013e3181f96282. PMID 20975057.
- ↑ 14.0 14.1 14.2 Fouladi, M.; Jenkins, J.; Burger, P.; Langston, J.; Merchant, T.; Heideman, R.; Thompson, S.; Sanford, A. et al. (Jul 2001). "Pleomorphic xanthoastrocytoma: favorable outcome after complete surgical resection.". Neuro Oncol 3 (3): 184-92. PMID 11465399.
- ↑ Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; Aster, Jon (2009). Robbins and Cotran pathologic basis of disease (8th ed.). Elsevier Saunders. pp. 1333. ISBN 978-1416031215.
- ↑ 16.0 16.1 Dias-Santagata, D.; Lam, Q.; Vernovsky, K.; Vena, N.; Lennerz, JK.; Borger, DR.; Batchelor, TT.; Ligon, KL. et al. (2011). "BRAF V600E mutations are common in pleomorphic xanthoastrocytoma: diagnostic and therapeutic implications.". PLoS One 6 (3): e17948. doi:10.1371/journal.pone.0017948. PMID 21479234.
- ↑ Cataltepe, O.; Turanli, G.; Yalnizoglu, D.; Topçu, M.; Akalan, N. (Apr 2005). "Surgical management of temporal lobe tumor-related epilepsy in children.". J Neurosurg 102 (3 Suppl): 280-7. doi:10.3171/ped.2005.102.3.0280. PMID 15881751.
- ↑ 18.0 18.1 18.2 O'Brien, DF.; Farrell, M.; Delanty, N.; Traunecker, H.; Perrin, R.; Smyth, MD.; Park, TS. (Dec 2007). "The Children's Cancer and Leukaemia Group guidelines for the diagnosis and management of dysembryoplastic neuroepithelial tumours.". Br J Neurosurg 21 (6): 539-49. doi:10.1080/02688690701594817. PMID 18071981.
- ↑ Grajkowska, W.; Kotulska, K.; Jurkiewicz, E.; Roszkowski, M.; Daszkiewicz, P.; Jóźwiak, S.; Matyja, E. (2011). "Subependymal giant cell astrocytomas with atypical histological features mimicking malignant gliomas.". Folia Neuropathol 49 (1): 39-46. PMID 21455842.
- ↑ 20.0 20.1 URL: http://path.upmc.edu/cases/case179.html. Accessed on: 29 July 2011.
- ↑ 21.0 21.1 Taraszewska, A.; Kroh, H.; Majchrowski, A. (1997). "Subependymal giant cell astrocytoma: clinical, histologic and immunohistochemical characteristic of 3 cases.". Folia Neuropathol 35 (3): 181-6. PMID 9595853.
- ↑ 22.0 22.1 URL: http://path.upmc.edu/cases/case179/micro.html. Accessed on: 8 January 2012.
- ↑ 23.0 23.1 23.2 23.3 23.4 Perry, Arie; Brat, Daniel J. (2010). Practical Surgical Neuropathology: A Diagnostic Approach: A Volume in the Pattern Recognition series (1st ed.). Churchill Livingstone. pp. 86. ISBN 978-0443069826.
- ↑ Komotar RJ, Mocco J, Jones JE, et al. (June 2005). "Pilomyxoid astrocytoma: diagnosis, prognosis, and management". Neurosurg Focus 18 (6A): E7. PMID 16048293.
- ↑ Alimohamadi M, Bidabadi MS, Ayan Z, Ketabchi E, Amirjamshidi A (December 2009). "Pilomyxoid astrocytoma with involvement of the sella turcica in an adolescent". J Clin Neurosci 16 (12): 1648–9. doi:10.1016/j.jocn.2009.01.035. PMID 19766001.
- ↑ 26.0 26.1 26.2 Wippold FJ, Lubner M, Perrin RJ, Lämmle M, Perry A (October 2007). "Neuropathology for the neuroradiologist: Antoni A and Antoni B tissue patterns". AJNR Am J Neuroradiol 28 (9): 1633–8. doi:10.3174/ajnr.A0682. PMID 17893219. http://www.ajnr.org/cgi/reprint/28/9/1633.
- ↑ Levy AD, Quiles AM, Miettinen M, Sobin LH (March 2005). "Gastrointestinal schwannomas: CT features with clinicopathologic correlation". AJR Am J Roentgenol 184 (3): 797–802. PMID 15728600. http://www.ajronline.org/cgi/content/full/184/3/797.
- ↑ URL: http://medical-dictionary.thefreedictionary.com/ganglioma. Accessed on: 8 November 2010.
- ↑ 29.0 29.1 Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B (July 1999). "Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee". Cancer 86 (2): 349–63. PMID 10421272.
- ↑ R. Kiehl. 8 November 2010.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 217. ISBN 978-0443066573.
- ↑ Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; Aster, Jon (2009). Robbins and Cotran pathologic basis of disease (8th ed.). Elsevier Saunders. pp. 1334. ISBN 978-1416031215.
- ↑ URL: http://emedicine.medscape.com/article/1744030-overview. Accessed on: 17 January 2012.
- ↑ http://www.pathology.vcu.edu/WirSelfInst/tumor-2.html
- ↑ MUN. 6 Oct 2009.
- ↑ 36.0 36.1 Singh, A.; Vermani, S.; Shruti, S.. "Choroid plexus carcinoma: report of two cases.". Indian J Pathol Microbiol 52 (3): 405-7. doi:10.4103/0377-4929.55009. PMID 19679976.
- ↑ 37.0 37.1 37.2 Menon, G.; Nair, SN.; Baldawa, SS.; Rao, RB.; Krishnakumar, KP.; Gopalakrishnan, CV.. "Choroid plexus tumors: an institutional series of 25 patients.". Neurol India 58 (3): 429-35. doi:10.4103/0028-3886.66455. PMID 20644273.
- ↑ PST. 14 February 2011.
- ↑ Buccoliero AM, Castiglione F, Degl'Innocenti DR, et al. (February 2010). "Embryonal tumor with abundant neuropil and true rosettes: morphological, immunohistochemical, ultrastructural and molecular study of a case showing features of medulloepithelioma and areas of mesenchymal and epithelial differentiation". Neuropathology 30 (1): 84–91. doi:10.1111/j.1440-1789.2009.01040.x. PMID 19563506.
- ↑ 40.0 40.1 40.2 40.3 Wippold FJ, Perry A (March 2006). "Neuropathology for the neuroradiologist: rosettes and pseudorosettes". AJNR Am J Neuroradiol 27 (3): 488–92. PMID 16551982.
- ↑ Ferri Niguez, B.; Martínez-Lage, JF.; Almagro, MJ.; Fuster, JL.; Serrano, C.; Torroba, MA.; Sola, J. (Aug 2010). "Embryonal tumor with abundant neuropil and true rosettes (ETANTR): a new distinctive variety of pediatric PNET: a case-based update.". Childs Nerv Syst 26 (8): 1003-8. doi:10.1007/s00381-010-1179-x. PMID 20499240.
- ↑ 42.0 42.1 Raoux D, Duband S, Forest F, et al. (June 2010). "Primary central nervous system lymphoma: Immunohistochemical profile and prognostic significance". Neuropathology 30 (3): 232–40. doi:10.1111/j.1440-1789.2009.01074.x. PMID 19925562.
- ↑ URL: http://moon.ouhsc.edu/kfung/jty1/Composites/FNA0IE14-Neurocytoma-Micro.htm. Accessed on: 12 October 2011.
- ↑ URL: http://path.upmc.edu/cases/case383/dx.html. Accessed on: 15 January 2012.
- ↑ 45.0 45.1 Chuang, MT.; Lin, WC.; Tsai, HY.; Liu, GC.; Hu, SW.; Chiang, IC.. "3-T proton magnetic resonance spectroscopy of central neurocytoma: 3 case reports and review of the literature.". J Comput Assist Tomogr 29 (5): 683-8. PMID 16163043.
- ↑ URL: http://moon.ouhsc.edu/kfung/jty1/Com/Com307-1-Diss.htm. Accessed on: 12 January 2012.
- ↑ Kerkeni, A.; Ben Lakhdher, Z.; Rkhami, M.; Sebai, R.; Belguith, L.; Khaldi, M.; Ben Hamouda, M. (Oct 2010). "[Central neurocytoma: Study of 32 cases and review of the literature].". Neurochirurgie 56 (5): 408-14. doi:10.1016/j.neuchi.2010.07.001. PMID 20692674.
- ↑ URL: http://moon.ouhsc.edu/kfung/jty1/Com/Com307-1-Diss.htm. Accessed on: 27 May 2011.
- ↑ Schmidt, MH.; Gottfried, ON.; von Koch, CS.; Chang, SM.; McDermott, MW. (Feb 2004). "Central neurocytoma: a review.". J Neurooncol 66 (3): 377-84. PMID 15015671.
- ↑ Im, SH.; Chung, CK.; Cho, BK.; Lee, SK. (Mar 2002). "Supratentorial ganglioglioma and epilepsy: postoperative seizure outcome.". J Neurooncol 57 (1): 59-66. PMID 12125968.
- ↑ Yağci-Küpeli, B.; Oguz, KK.; Bilen, MA.; Yalçin, B.; Akalan, N.; Büyükpamukçu, M. (Mar 2010). "An unusual cause of posterior fossa mass: Lhermitte-Duclos disease.". J Neurol Sci 290 (1-2): 138-41. doi:10.1016/j.jns.2009.12.010. PMID 20060133.