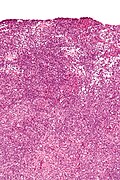
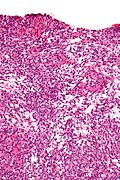
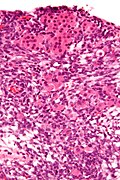
Sertoli-Leydig cell tumour
Sertoli-Leydig cell tumour, also Sertoli-Leydig tumour, is a rare tumour of the gonad in the sex cord-stromal group of tumours.
General
- Sertoli and leydig cells are normal in the testis.
- Tumor was called androblastoma or arrhenoblastoma in the past
- May present with masculinization (virilization).[1]
- May present as abdominal swelling or pain.
- Generally a tumor of younger women and can present in children.[2]
- 75% younger than 30 years of age
- 10% over 50 years of age.
Microscopic
Features:
- Sertoli or Leydig cells.[3]
- Leydig cells:
- Polygonal pink cells
- Abundant solid or somewhat granular eosinophilic cytoplasm.
- Round nuclei with fine chromatin and a small or indistinct nucleolus.
- Often in small clusters ~ 5-25 cells/cluster.
- Sertoli cells:
- Pale/clear vacuolated cytoplasm.
- Irregular nuclei with irregular/vacuolated-appearing chromatin.
- Architecture: tubules, cords or sheets.
- Classic Sertoli tubule shows an 'antipodal arrangement of nuclei'
- Nuclei sit near the basement membrane away from the tubule lumen.
- A fair bit of cytoplasm sits above the nucleus.
- Lumen is round.
- Classic Sertoli tubule shows an 'antipodal arrangement of nuclei'
- Mitotic activity may be much lower than expected for the degree of atypia (in comparison to adenocarcinoma).
- Leydig cells:
- Growth Patterns:
- Well-differentiated.
- Hollow or solid tubules of mature Sertoli cells with Leydig cells in the intervening stroma.
- Intermediate (most common).
- Jumbled admixture of dark blue Sertoli cells and Leydig cells.
- Lobules comprising sheets of Sertoli cells.
- Some areas of tubules.
- Poorly differentiated.
- Masses of malignant spindle cells – sheets of cells can be reminiscent of fibrosarcoma or granulosa cell tumour.
- Tubules may be a very minor element.
- Poorly differentiated tumours have sarcomatous features.[3]
- With heterologous element.
- Mucinous intestinal-type epithelium, cartilage, skeletal muscle.
- Heterologous elements can also occur with retiform or poorly differentiated tumours.
- Retiform.[4]
- Tumour resembles rete testis/ovary with an irregular network of elongated slit-like tubules and cysts, which may contain papillae.
- Well-differentiated.
- +/-Stromal edema may be prominent
- +/-Sarcomatous features (mucinous glands, bone, cartilage).
DDx:
- Endometrioid carcinoma of the ovary (sertoliform variant)
- Should be positive for EMA, CK7 and negative for inhibin and calretinin.[5]
- Should have some characteristic areas of endometriod carcinoma and may have some typical features
- Cilia, squamous metaplasia, mucin production
- Luteinized adult granulosa cell tumour - super rare, 50% of cell with eosinophilic cytoplasm, other findings of granulosa cell tumour, e.g. Call-Exner bodies. More likely to be keratin negative than a Sertoli-Leydig cell tumor. [6]
- Ovarian carcinosarcoma - especially considering poorly differentiated versions with heterologous areas.
Retiform variant
- Ovarian serous carcinoma - generally younger patients than usual for this diagnosis
- Ovarian yolk sac tumor
Images
www:
IHC
Features:[7]
- Inhibin +ve
- Calretinin +ve.
- WT-1 +ve.
- Melan A (MART-1) +ve - marks the Leydig component.
- Vimentin +ve.[8]
- CD99 +ve.
- AE1/AE3 and PanKeratin +ve
Others:[8]
- CD34 -ve.
- EMA -ve.
Keep in mind that this is a biphasic tumor - Leydig cells will not be Pan-keratin positive - Sertoli cells do not express calretinin - Both components express inhibin - etcetera - interpreting this immunopanal requires correlation with the histomorphology.
Pan-keratins and AE1/AE3 may mark granulosa cell tumors and Sertoli cell tumors causing confusion with adenocarcinoma. EMA is a better marker to exclude an epithelial tumor as EMA is negative in sex cord-stromal tumors. Highlighting why a panel of stains is needed, endometrioid adenocarcinomas may occasionally weakly express inhibin, calretinin or WT-1.
See also
References
- ↑ Xiao, H.; Li, B.; Zuo, J.; Feng, X.; Li, X.; Zhang, R.; Wu, L. (Mar 2013). "Ovarian Sertoli-Leydig cell tumor: a report of seven cases and a review of the literature.". Gynecol Endocrinol 29 (3): 192-5. doi:10.3109/09513590.2012.738723. PMID 23173550.
- ↑ Young, RH.; Scully, RE. (Aug 1985). "Ovarian Sertoli-Leydig cell tumors. A clinicopathological analysis of 207 cases.". Am J Surg Pathol 9 (8): 543-69. PMID 3911780.
- ↑ 3.0 3.1 Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 1103. ISBN 0-7216-0187-1.
- ↑ Young, RH.; Scully, RE. (Dec 1983). "Ovarian Sertoli-Leydig cell tumors with a retiform pattern: a problem in histopathologic diagnosis. A report of 25 cases.". Am J Surg Pathol 7 (8): 755-71. PMID 6660351.
- ↑ McCluggage, WG.; Young, RH. (Apr 2007). "Ovarian sertoli-leydig cell tumors with pseudoendometrioid tubules (pseudoendometrioid sertoli-leydig cell tumors).". Am J Surg Pathol 31 (4): 592-7. doi:10.1097/01.pas.0000213365.56498.72. PMID 17414107.
- ↑ Ganesan, R.; Hirschowitz, L.; Baltrušaitytė, I.; McCluggage, WG. (Sep 2011). "Luteinized adult granulosa cell tumor--a series of 9 cases: revisiting a rare variant of adult granulosa cell tumor.". Int J Gynecol Pathol 30 (5): 452-9. doi:10.1097/PGP.0b013e318214b17f. PMID 21804396.
- ↑ Zhao, C.; Vinh, TN.; McManus, K.; Dabbs, D.; Barner, R.; Vang, R. (Mar 2009). "Identification of the most sensitive and robust immunohistochemical markers in different categories of ovarian sex cord-stromal tumors.". Am J Surg Pathol 33 (3): 354-66. doi:10.1097/PAS.0b013e318188373d. PMID 19033865.
- ↑ 8.0 8.1 Kondi-Pafiti, A.; Grapsa, D.; Kairi-Vassilatou, E.; Carvounis, E.; Hasiakos, D.; Kontogianni, K.; Fotiou, S. (2010). "Granulosa cell tumors of the ovary: a clinicopathologic and immunohistochemical study of 21 cases.". Eur J Gynaecol Oncol 31 (1): 94-8. PMID 20349790.