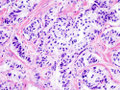
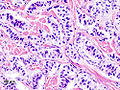
Neuroendocrine tumour of the pancreas
| Neuroendocrine tumour of the pancreas | |
|---|---|
| Diagnosis in short | |
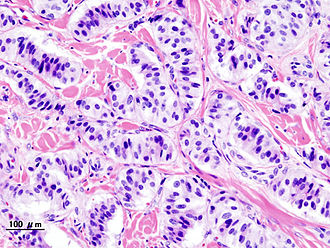 Pancreatic neuroendocrine tumour (insulinoma). H&E stain. | |
|
| |
| Synonyms | pancreatic islet cell tumour (obsolete term) |
|
| |
| LM | nests of cells or cords, stippled chromatin, moderate quantity of cytoplasm, +/-hyaline globules |
| LM DDx | solid pseudopapillary neoplasm, acinar cell carcinoma, invasive ductal carcinoma of the pancreas |
| IHC | chromogranin +ve, synaptophysin +ve, insulin +/-ve, glucagon +/-ve, gastrin +/-ve, somatostatin +/-ve |
| Site | pancreas |
|
| |
| Syndromes | Multiple endocrine neoplasia I, von Hippel-Lindau disease, neurofibromatosis type 1 |
|
| |
| Symptoms | dependent on subtype |
| Prevalence | uncommon |
| Radiology | pancreatic mass |
| Clin. DDx | invasive ductal carcinoma of the pancreas, other pancreatic tumours |
Neuroendocrine tumour of the pancreas, also pancreatic neuroendocrine tumour, is a relatively uncommon tumour.
It may be abbreviated PanNET.[1]
Previously, it was referred to as pancreatic islet cell tumour or islet cell tumour; these terms are now considered to be outdated.[1]
Neuroendocrine tumours in general are dealt with in the neuroendocrine tumours article.
General
- Rare ~ 1% of pancreatic tumours.[2]
- Presentation depends on subtype, e.g. for insulinoma the typical presentation is hypoglycemia.
- May be part of a syndrome:
Classification
Based on peptide produced in the pancreatic islets:
- Glucagon from alpha cells (glucagonoma).
- Insulin from beta cells (insulinoma) - most common ~ 50% of islet cell tumours.
- Somatostatin from D cells (somatostatinoma).
- Pancreatic polypeptide from PP cells.
Others:
- Vasoactive intestinal peptide (VIPoma).
- Gastrin (gastrinoma).
- May be seen in Zollinger-Ellison syndrome.
- Triad: pancreatic gastrinoma, gastric acid hypersecretion, marked peptic ulcers in the small bowel.[5]
- May be seen in Zollinger-Ellison syndrome.
Gross
Microscopic
Features:
- Nests of cells or cords.
- Stippled chromatin - key feature.
- Moderate cytoplasm.
- +/-Hyaline globules.
DDx:
Images
www:
- Islet cell tumour (upmc.edu).
- Pancreatic NET with features of SPT (upmc.edu).
- Pancreatic NET - another case (upmc.edu).
IHC
- CK19 +ve -- should be done as a routine in pancreatic NETs; poor prognostic factor.[7]
- Chromogranin +ve.
- Synaptophysin +ve.
- CD56 +ve.
A panel:
- Chromogranin, synaptophysin, CD10, PR, beta-catenin, CK7, pankeratin, Ki-67, CK19.
Note:
- CK19 should not be confused with CA19-9.
Sign out
TAIL OF PANCREAS AND SPLEEN, PARTIAL PANCREATECTOMY AND SPLENECTOMY: - CYSTIC PANCREATIC NEUROENDOCRINE TUMOUR, pT1, pN0. -- INTERMEDIATE GRADE (G2). -- NARROWLY EXCISED. -- PLEASE SEE TUMOUR SUMMARY. - UNREMARKABLE SURROUNDING PANCREAS WITH FAT. - SPLEEN WITHIN NORMAL LIMITS. - FOUR BENIGN LYMPH NODES.
TAIL OF PANCREAS AND SPLEEN, PARTIAL PANCREATECTOMY AND SPLENECTOMY: - CYSTIC PANCREATIC NEUROENDOCRINE TUMOUR, pT1, pN0. -- LOW GRADE (G1). -- SURGICAL MARGINS NEGATIVE. -- PLEASE SEE TUMOUR SUMMARY. - UNREMARKABLE SURROUNDING PANCREAS WITH FAT. - SPLEEN WITHIN NORMAL LIMITS. - FOUR BENIGN LYMPH NODES.
Micro
The sections show pancreas with a large well-circumscribed tumour that centrally has a large cyst-like cavity. The tumour cells have moderate pale grey cytoplasm and round nuclei with salt and pepper chromatin. The tumour cells are arranged in cords and nests. No cholesterol clefts are readily apparent. No hyaline globules are identified. No papillary structures are apparent.
No necrosis is identified. No degenerative changes are apparent. Mitotic activity is seen focally. There are 2 mitoses per 10 high power fields, were one high power field has an area of 0.2376 mm*mm.
See also
References
- ↑ 1.0 1.1 Burns, WR.; Edil, BH. (Dec 2011). "Neuroendocrine Pancreatic Tumors: Guidelines for Management and Update.". Curr Treat Options Oncol. doi:10.1007/s11864-011-0172-2. PMID 22198808.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 496. ISBN 978-0443066573.
- ↑ Charlesworth, M.; Verbeke, CS.; Falk, GA.; Walsh, M.; Smith, AM.; Morris-Stiff, G. (Feb 2012). "Pancreatic Lesions in von Hippel-Lindau Disease? A Systematic Review and Meta-synthesis of the Literature.". J Gastrointest Surg. doi:10.1007/s11605-012-1847-0. PMID 22370733.
- ↑ Alexakis, N.; Connor, S.; Ghaneh, P.; Lombard, M.; Smart, HL.; Evans, J.; Hughes, M.; Garvey, CJ. et al. (2004). "Hereditary pancreatic endocrine tumours.". Pancreatology 4 (5): 417-33; discussion 434-5. doi:10.1159/000079616. PMID 15249710.
- ↑ Zollinger RM, Ellison EH (1955). "Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas". Ann. Surg. 142 (4): 709–23; discussion, 724–8. doi:10.1097/00000658-195510000-00015. PMC 1465210. PMID 13259432. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1465210/.
- ↑ 6.0 6.1 Oh, TG.; Chung, MJ.; Park, JY.; Bang, SM.; Park, SW.; Chung, JB.; Song, SY. (Sep 2012). "Prognostic factors and characteristics of pancreatic neuroendocrine tumors: single center experience.". Yonsei Med J 53 (5): 944-51. doi:10.3349/ymj.2012.53.5.944. PMC 3423842. PMID 22869477. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3423842/. Cite error: Invalid
<ref>tag; name "pmid22869477" defined multiple times with different content - ↑ Jain, R.; Fischer, S.; Serra, S.; Chetty, R. (Jan 2010). "The use of Cytokeratin 19 (CK19) immunohistochemistry in lesions of the pancreas, gastrointestinal tract, and liver.". Appl Immunohistochem Mol Morphol 18 (1): 9-15. doi:10.1097/PAI.0b013e3181ad36ea. PMID 19956064.