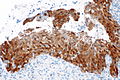
High-grade squamous intraepithelial lesion
| High-grade squamous intraepithelial lesion | |
|---|---|
| Diagnosis in short | |
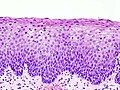
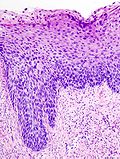
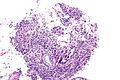
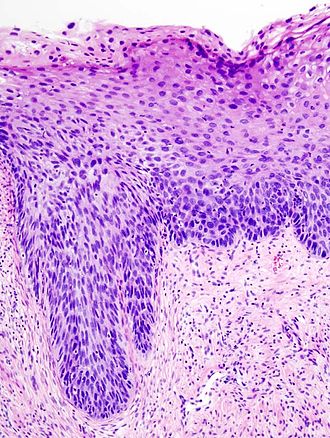 HSIL. H&E stain. | |
|
| |
| LM | nuclear membrane irregularies, nuclear hyperchromasia, +/-clear perinuclear halos (koilocytic change), +/-nuclear enlargement, large NC ratio (usually ~ 1:3), +/-mitoses (lower third) |
| LM DDx | low-grade squamous intraepithelial lesion, squamous metaplasia, reactive changes, normal cervix, atrophy of the uterine cervix |
| Site | uterine cervix |
|
| |
| Signs | acetowhite lesion |
| Prevalence | common |
| Clin. DDx | squamous metaplasia, LSIL |
High-grade squamous intraepithelial lesion, abbreviated HSIL, is a pre-cancerous lesions of the uterine cervix.
Increasingly, the term is being applied to other anatomical sites, e.g. vagina.
It is in the larger category of squamous intraepithelial lesion, abbreviated SIL.
General
- Precursor lesion of cervical squamous cell carcinoma.
- Usually associated with human papilloma virus.
Divided into grades:
- Low-grade.
- High-grade.
The new and old terminology
| SIL (current terminology) | LSIL | HSIL |
|---|---|---|
| Recent terminology | CIN I | CIN II, CIN III |
| Very old terminology | mild dysplasia | moderate dysplasia, severe dysplasia |
Treatment
Overview:
Procedures
Loop electrosurgical excision procedure (LEEP):
- Used for squamous lesions -- pathologist typically gets several pieces.
Cone:
- Used for endocervical lesions, i.e. adenocarcinoma in situ (AIS).
- Pathologist gets a ring or donut-shaped piece of tissue.
Gross
- Acetowhite lesion at colposcopy.
Microscopic
Features:[1]
- Increased nuclear-cytoplasmic ratio, loss of polarity, incr. mitoses, hyperchromasia.
- If there are large nuclei... you should seen 'em on low power, i.e. 25x.
Notes:
- Hyperchromasia is a very useful feature for identifying SIL (particularly at low power, i.e. 25x).
- Koilocytes are the key feature of LSIL.
- Koilocytes are not typically seen in HSIL.
- Large irregular nuclei are not required for HSIL... but you should think about it.
- Some mild changes at the squamo-columnar junction are expected.
- Look for the location of mitoses...
- If there is a mitosis in the inner third (of the epithelial layer) = think LSIL.
- If there is a mitosis in the middle third (of the epithelial layer) = think HSIL (CIN II).
- If there is a mitosis in the outer third = think HSIL (CIN III).
- Prominent nucleoli are not present in HSIL.[2]
- Nucleoli are common in reactive changes.[3]
- The most probably place for SIL is the posterior cervix (6 o'clock position) - risk is marginally increased.[4]
- The difference between CIN II (moderate dysplasia) and CIN III (severe dysplasia) is: changes as in CIN II + outer third (or full thickness).[1]
DDx:
- Low-grade squamous intraepithelial lesion.
- Uterine cervix with atrophic changes.
- Squamous cell carcinoma of the uterine cervix.
Images
Image:
www:
- CIN III (flickr.com/euthman).
- CIN III (flickr.com/euthman).
- CIN III - several images (flickriver.com).
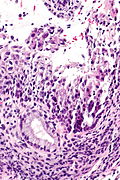
IHC
Features:[5]
- p16.
- Diffuse strong staining involving at least all of the basal aspect of the epithelium = CIN II or CIN III.
- Patchy, weak positive staining = CIN I or squamous metaplasia.
- Ki-67.
- Several positive cells above basal layer suggests CIN II or CIN III.
Notes:
- Both p16 and Ki-67 are usually negative in CIN I -- 75% of cases.[6]
- CIN I with p16 staining appears to have a higher risk of progression the p16 negative CIN I.[7]
Sign-out
LEEP
UTERINE CERVIX, LOOP ELECTROSURGICAL EXCISION PROCEDURE (LEEP): - CERVICAL INTRAEPITHELIAL NEOPLASIA 2 (MODERATE DYSPLASIA). - DEEP, ENDOCERVICAL AND EXOCERVICAL MARGINS NEGATIVE FOR INTRAEPITHELIAL NEOPLASIA.
UTERINE CERVIX, LOOP ELECTROSURGICAL EXCISION PROCEDURE (LEEP): - CERVICAL INTRAEPITHELIAL NEOPLASIA 3 (SEVERE DYSPLASIA). - DEEP, ENDOCERVICAL AND EXOCERVICAL MARGINS NEGATIVE FOR INTRAEPITHELIAL NEOPLASIA.
UTERINE CERVIX, LOOP ELECTROSURGICAL EXCISION PROCEDURE (LEEP): - CERVICAL INTRAEPITHELIAL NEOPLASIA 3 (SEVERE DYSPLASIA). - DEEP, ENDOCERVICAL AND EXOCERVICAL MARGINS NEGATIVE FOR INTRAEPITHELIAL NEOPLASIA. - NEGATIVE FOR MALIGNANCY. COMMENT: CIN 3 is seen in 2 of 5 blocks and has a total linear extent of 17 millimeters.
UTERINE CERVIX, LOOP ELECTROSURGICAL EXCISION PROCEDURE (LEEP): - HIGH-GRADE SQUAMOUS INTRAEPITHELIAL LESION (HSIL). - DEEP, ENDOCERVICAL AND EXOCERVICAL MARGINS NEGATIVE FOR DYSPLASIA. - NEGATIVE FOR MALIGNANCY. COMMENT: HSIL is seen in 3 of 4 blocks and has a total linear extent of approximately 12 millimeters. The HSIL is in keeping with cervical intraepithelial neoplasia 3 (severe dysplasia).
Cervical biopsy
At least CIN 2
UTERINE CERVIX, BIOPSY: - AT LEAST CERVICAL INTRAEPITHELIAL NEOPLASIA 2 (MODERATE DYSPLASIA). - TRANSFORMATION ZONE PRESENT.
UTERINE ENDOCERVIX, CURETTAGE: - HIGH-GRADE SQUAMOUS INTRAEPITHELIAL LESION (HSIL). - ENDOCERVICAL MUCOSA AND STRIPPED ENDOCERVICAL EPITHELIUM WITHIN NORMAL LIMITS. COMMENT: The HSIL is in keeping with CIN 2.
UTERINE CERVIX, BIOPSY: - HIGH-GRADE SQUAMOUS INTRAEPITHELIAL LESION (HSIL). - TRANSFORMATION ZONE PRESENT. COMMENT: A p16 immunostain is strong and within the limits of the tissue orientation appears to mark the full thickness of squamous epithelium in the suspicious area. A Ki-67 immunostain marks increased numbers of superficial epithelial cells. The HSIL is in keeping with at least CIN 2.
CIN 2 surrounded by endocervical epithelium
UTERINE CERVIX, BIOPSY: - HIGH-GRADE SQUAMOUS INTRAEPITHELIAL LESION (HSIL). - ENDOCERVICAL EPITHELIUM WITHIN NORMAL LIMITS. COMMENT: A p16 stain marks the full thickness of the squamous epithelium and is strong. A Ki-67 stain marks increased numbers of superficial epithelial cells. The epithelium surrounding the lesion is endocervical. No normal exocervical epithelium is apparent in the sampled tissue. The HSIL is in keeping with CIN 2.
CIN 3
UTERINE CERVIX, BIOPSY: - CERVICAL INTRAEPITHELIAL NEOPLASIA 3 (SEVERE DYSPLASIA).
COMMENT: A p16 stain marks the full thickness of the squamous epithelium and is strong. A Ki-67 stain marks increased numbers of superficial epithelial cells.
Micro
CIN 2
The sections show the transformation zone.
The squamous epithelium has a moderately increased nuclear-to-cytoplasmic ratio, and nuclear hyperchromasia extending to the mid level of the epithelium. Binucleation is present. A mid level mitoses is seen in one section.
A p16 immunostain, within the limits of the tissue orientation, focally marks (in the suspicious area) weakly the full thickness of the squamous epithelium and strongly marks up to the lower half of the epithelium. A Ki-67 immunostain marks increased numbers of superficial epithelial cells.
CIN 3
The sections show the transformation zone.
The squamous epithelium has an increased nuclear-cytoplasmic ratio, loss of polarity, mitoses and nuclear hyperchromasia extending to the superficial third of the epithelium. Mitoses are seen in the upper third of the epithelium. No nucleoli are present. No invasion is identified.
The columnar epithelium has focal involvement by the squamous lesion. There is no columnar dysplasia. The margins are negative for dysplasia.
Biopsy
The sections show the transformation zone.
The squamous epithelium has an increased nuclear-cytoplasmic ratio, loss of polarity, mitoses and nuclear hyperchromasia extending to the superficial third of the epithelium. Mitoses are seen in the upper third of the epithelium. Nucleoli are not apparent. No invasion is identified.
No columnar dysplasia is identified.
Alternate
The sections show fragments of transformation zone.
There is dysplastic squamous epithelium with coarse chromatin, nuclear hyperchromasia, nuclear enlargement, irregular nuclear membranes, and an increase nuclear-to-cytoplasmic ratio. Mitotic activity is abundant focally (5 mitoses/0.2376 mm*mm). The dysplastic squamous epithelium does not show appreciable maturation toward the surface (CIN 3). The dysplastic squamous epithelium is not associated with stroma; thus, the presence/absence of invasion cannot be assessed. Small nucleoli are seen rarely.
There is benign squamous epithelium. Scant benign stripped endocervical epithelium is present.
See also
References
- ↑ 1.0 1.1 Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 1075-6. ISBN 0-7216-0187-1.
- ↑ Nucci, Marisa R.; Oliva, Esther (2009). Gynecologic Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 146. ISBN 978-0443069208.
- ↑ STC. January 2009.
- ↑ Pretorius, RG.; Zhang, X.; Belinson, JL.; Zhang, WH.; Ren, SD.; Bao, YP.; Qiao, YL. (Jan 2006). "Distribution of cervical intraepithelial neoplasia 2, 3 and cancer on the uterine cervix.". J Low Genit Tract Dis 10 (1): 45-50. PMID 16378031.
- ↑ Singh, M.; Mockler, D.; Akalin, A.; Burke, S.; Shroyer, A.; Shroyer, KR. (Feb 2012). "Immunocytochemical colocalization of P16(INK4a) and Ki-67 predicts CIN2/3 and AIS/adenocarcinoma.". Cancer Cytopathol 120 (1): 26-34. doi:10.1002/cncy.20188. PMID 22162342.
- ↑ Jackson, JA.; Kapur, U.; Erşahin, Ç. (Apr 2012). "Utility of p16, Ki-67, and HPV test in diagnosis of cervical intraepithelial neoplasia and atrophy in women older than 50 years with 3- to 7-year follow-up.". Int J Surg Pathol 20 (2): 146-53. doi:10.1177/1066896911427703. PMID 22104735.
- ↑ del Pino, M.; Garcia, S.; Fusté, V.; Alonso, I.; Fusté, P.; Torné, A.; Ordi, J. (Nov 2009). "Value of p16(INK4a) as a marker of progression/regression in cervical intraepithelial neoplasia grade 1.". Am J Obstet Gynecol 201 (5): 488.e1-7. doi:10.1016/j.ajog.2009.05.046. PMID 19683687.