Difference between revisions of "Stains"
Jensflorian (talk | contribs) (→Verhoeff-van Gieson stain: +image) |
Jensflorian (talk | contribs) m (→Luxol fast blue stain: images) |
||
| Line 285: | Line 285: | ||
<gallery> | <gallery> | ||
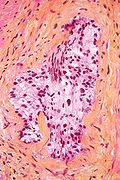
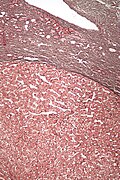
Image:Globus_pallidus_and_putamen_-_very_low_mag.jpg | Globus pallidus and putamen - H&E-LFB. (WC) | Image:Globus_pallidus_and_putamen_-_very_low_mag.jpg | Globus pallidus and putamen - H&E-LFB. (WC) | ||
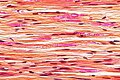
File:LFB_CNS_cortex_supratentorial.jpg | Normal cortex - LFB only. (WC/jensflorian) | |||
File:LFB_CNS_cortex_grey-white_matter_junction.jpg | White-grey matter junction - LFB. (WC/jensflorian) | |||
</gallery> | </gallery> | ||
==Giemsa stain== | ==Giemsa stain== | ||
===Use=== | ===Use=== | ||
Revision as of 12:22, 21 July 2015

This article deals with stains. H&E isn't the only stain out there.
Non-H&E stains are often referred to as special stains.
Where to start...
Principles
When considering additional (i.e. special) stains one should (in order) do the following:[1]
- Make sure one has exhausted the clinical history; history is considered the best special stain.
- Special stains (below).
- Immunohistochemistry (dealt with in a separate article).
- Molecular testing, electron microscopy.
Common stains
- H&E stain.
- PAS stain.
- PAS-D stain.
- AFB stains, e.g. Ziehl-Neelsen stain.
- Congo red.
- GMS stain.
- Gram stain.
Immunohistochemistry
General
- Abbreviated IHC.
Interpretation
Simple version:
- Positive is (usually): brown.
- Negative tissue is: light blue.
Important notes:
- One has to know where the target (of the antibody) is supposed to be, i.e. cytoplasm vs. cell membrane.
- The edge of the tissue may have light staining - edge effect.
- If everything is brown... suspect that it didn't work.
- In some situations you're blessed with an internal control, e.g. in renal tumours CD10 will stain RCC and the proximal tubule, in GISTs - CD117 the mast cells are positive.
Work-up of infection
It often not possible to be definitive by staining.[2]
Basic panel:
- Gram stain - for bacteria.
- GMS stain - fungal stain.
- PAS (or PAS-D) - fungal stain.
Fungi
Fungi are a type of microorganisms. They are seen by pathologist every once in a while.
Specific stains
What follows is a big list... of stains.
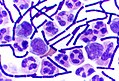
Haematoxylin and eosin stain
General
- Abbreviated H&E.
- Standard bearer in most pathology departments.
Intepretation
- Blue (haematoxylin) = nucleus.
- Pink (eosin) = cytoplasm.
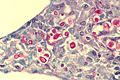
Images
Basal cell carcinoma. H&E stain. (WC)
Haematoxylin phyloxin saffron stain
General
- Abbreviated HPS.
- An alternative to the H&E stain - some pathol. departments use this as their standard.
Interpretation
- Haematoxylin = blue -- stains nucleus.
- Phyloxin = pink -- stains muscle and cytoplasm.
- Saffron = yellow -- stains collagen.
- An alternative to H&E stain.
- Fibrosis is easier to see on HPS than H&E... as one can see the collagen.
Images
Periodic acid Schiff stain
- Abbreviated PAS.
Primary application
- Kidney biopsies, medical.
- Liver biopsies, medical.
- Positive in alpha-1 antitrypsin deficiency.
Utility
- Stains - lipofuscin,[3] basement membranes, fungi, glycogen, (neutral) mucin.
Interpretation
- Magenta = glycogen, mucin, fungi.
- Blue = nuclei.
Ref.:[4]
Image
Periodic acid Schiff fungal stain
- Abbreviated PASF.
Primary application
- Look for fungal organisms.
Interpretation
- Light purple = fungi.
- Light green = background.
- Washed-out light purple = Gram positive bacilli.
Note:
- This is much improved over the PAS in the context of skin, as the background is similar to the fungal organisms.
Periodic acid Schiff with diastase
- Abbreviated: PAS-D and PASD.
General
Use
- Stains mucin.
- Used to identify glycogen (together with PAS stain).
- Glycogen = clear (digested) on PAS-D.
- Glycogen = magenta on PAS.
Notes: [6]
Interpretation
- Light purple = fungi.
- Light blue/pink = background. ???
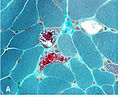
Gomori methenamine-silver stain
- Abbreviated GMS.
Note:
- GMS is "Grocott's methenamine Silver" according to WMSP.[7]
Use
- Useful for fungi.
- Pneumocystis jirovecii - cause of pneumocystis pneumonia (PCP).
- Histoplasma - cause of histoplasmosis.
- Histoplasma = black, round balls.
Image
Acid-fast bacilli stains
- Abbreviated: AFB.
There are several AFB stains:
- Ziehl-Neelson stain - used to look for Mycobacterium tuberculosis.
- Fite stain - used to look for Mycobacterium leprae.[8]
- Auramine-rhodamine stain.
Ziehl-Neelsen stain
- Most popular acid-fast bacilli stain.
- Stains other mycobacteria -- not specific for tuberculosis.
- Stains Nocardia.[9]
Image
Fite stain
Interpretation:
- Red = AFB.
- Blue = background.
Auramine-rhodamine stain
- Fluorescent stain.
Image
Kinyoun stain
- Another AFB stain[10] - useful for cryptosporidiosis and microsporidiosis.[11]
Congo red stain
Use
- Used to look for amyloid.
- Mnemonic: CRAP = congo red amyloid protein.
- An alternate stain for amyloid is Thioflavin T.
Note:
- Thick sections (~10 micrometers) are considered a requirement for the stain to work properly.[12]
- If the section is too thin... it doesn't work.
Interpretation
- Amyloid = pink/red.
- Nuclei = blue.
Ref.:[13]
Image
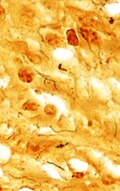
Congo red staining in cerebral amyloid angiopathy. (WC)
Thioflavin T stain
Use
- Used to look for amyloid.
Interpretation
- Amyloid = green.
Image: Amyloid (inano.au.dk).
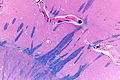
Gram stain
Use
- "It is useless for finding bacteria."[14]
- If they are to be seen... they'll be visible on H&E.
Note:
- Microbiology is better at finding organisms than pathology.
- They have one significant advantage -- if a small amount of bugs are present... they grows into a large (obviously visible) colony.
DDx for common patterns
A short list of bacteria and their characteristics:[15]
| Shape\Gram stain | Positive | Negative | Variable or negative |
|---|---|---|---|
| Bacilli | Clostridium difficile, Bacillus anthracis, Nocardia spp. | Escherichia coli, Helicobacter pylori, Yersinia pestis, Hemophilus influenzae | Mycobacterium tuberulosis, Legionella pneumophila[16] |
| Cocci | Streptococcus pneumoniae, Staphylococcus aureus | Neisseria meningitidis, Moraxella catarrhalis |
Interpretation
- Purple (or blue) = Gram positive organisms.
- Red = Gram negative organisms, nuclei.[17]
- Yellow = background.
Notes:
- Many of the bacteria are quite small relative to lymphocytes; Escherichia coli is 1-2 micrometers long x 0.25 micrometers in diameter.[18]
- Epithelial cell nuclei & stromal cell nuclei may stain red.
- Memory device: purple = positive.
Images
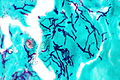
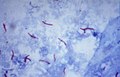
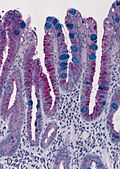
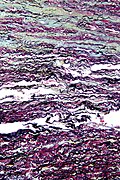
Luxol fast blue stain
- Abbreviated LFB.
Use
- Neuropathology, myelin stain.
Intepretation
- Blue = myelinated fibers (contain lipoproteins), lipofuscin.[19]
- Lack of blue (where it ought to be) = demyelination.
- Purple = nerve cell (e.g. neuron).
- Neutrophils = pink.
Ref.:[20]
Image
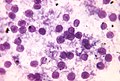
Giemsa stain
Use
- Useful for finding mast cells.
- Useful for finding Donovan bodies and Leishmania.[21]
Interpretation
- Tissue is light blue/green.
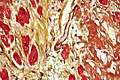
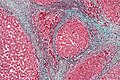
Reticulin stain
Use
- Liver biopsy, medical.
- Demonstrates the reticular fibers (in cirrhosis the fibers are disrupted).
- Before IHC, reticulin was used to differentiate sarcomas from carcinomas:[22]
- Sarcomas have reticulin around each cell.
- Carcinomas have reticulin around clusters of cells.
- Commonly used in neuropathology.
- In adenoma, reticulin highlights the lost acinar structure of normal pituitary gland.
- Paraganglioma (Zellballen architecture)
- Separating schwannoma (basement membrane around each cell) from meingioma in cerebellopontine angle.
- Separating desmoplastic medulloblastoma from classic/anaplastic forms.
Interpretation
- Black = reticular fibers.
- Red = nuclei.
Notes:[23]
Images
Liver. Reticulin stain. (WC)
Hepatic adenoma. Reticulin stain. (WC)
Cresyl violet stain
Use
- Used at some places (e.g. SMH) to look for Helicobacter organisms.
Interpretation
- Everything is shades of blue.
- Helicobacter stains blue.
Prussian blue stain
- AKA Perl's iron stain.
Use
- Useful for iron and hemosiderin; useful for differentiating brown pigments (melanin, lipofuscin, tattoo pigment, hemosiderin).
Interpretation
- Blue = iron.
Image:
Notes:
- Described well by vetmed.vt.edu.[24]
- DDx of brown pigment: Fontana-Masson (melanin), Kluver-Barrera stain (lipofuscin).
Images
Liver hemosiderosis. Prussian blue stain. (WC/Nephron)
Kluver-Barrera stain
Combination of:
- Luxol Fast Blue,
- Cresyl Violet,
- Special component for lipofuscin.
Use
- Useful for differentiating brown pigments (melanin, lipofuscin, tattoo pigment, hemosiderin).
- Stains lipofuscin.
- Useful to detect demyelinating lesions in the CNS.
Notes:
- PAS also stains lipofuscin and is more commonly available.
Interpretation
- Blue pigmented granules = lipofuscin.
Notes:
- Described well by vetmed.vt.edu.[25]
- DDx of brown pigment: Fontana-Masson (melanin), Prussian blue stain (hemosiderin).
Oil red O stain
Use
- Stains adipose tissue.
- Uncommon.
Notes:
- Must be done on fresh tissue, i.e. it cannot be fixed in formalin.
Interpretation
- Red = fat.
Images
Warthin-Starry stain
Background:
- Developed by a bunch of pathologists in Michigan to look for spirochetes.[26]
Use
- Find spirochetes, e.g. syphilis (Treponema pallidum),[27] cat-scratch disease (Bartonella henselae).
- Find Helicobacter spp., e.g. Helicobacter pylori -- Mount Sinai Hospital.[28]
Interpretation:[29]
- Spirochetes - black.
- Background - yellow.
Image
Notes:
- Considered a "dirty" stain - picks-up junk in the background.[30]
Dieterle stain
Considered a variant of the Steiner stain.[31]
Use
- Find spirochetes, e.g. syphilis (Treponema pallidum),[32] donovan bodies (leishmaniasis),[33] Helicobacter pylori and Bartonella henselae (Cat-scratch disease).[34]
Interpretation
- Spirochetes - black.
- Background - yellow.
Images
www:
Bielschowsky stain
Abbreviated: Biel stain.
Use
- Stains glial tissue, i.e. brain.
- Demonstrates neurofibrillary tangles, senile plaques (as in Alzheimer's disease).
Interpretation
- Black = axons, tangles, plaques.
- Brown/dark brown = plaque, vascular amyloid.
- Yellow/brown = other.
Ref.: [35]
Image
Mucicarmine stain
- Stains some mucins... uses the dye carmine.
Use
- Identify mucin.
- Malignant cells that produce mucin... carcinomas.[36]
Interpretation
- Carmine with metanil yellow and Weigert's Hematoxylin:[37]
- Blue/black = nucleus.
- Yellow = background.
- Red = mucin.[38]
Images
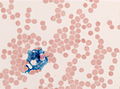
Cryptococcosis. Mucicarmine stain. (WC/CDC)
www:
- Mucicarmine stained bowel (medschool.lsuhsc.edu).
- Mucicarmine stained pancreatic adenosquamous carcinoma (nature.com).
Alcian blue stain
General
- Stains acidic mucin (pH=2.5); Alcian blue = Acidic.
- A variant uses pH=1.0.[7]
Note:
- Alcian blue (not otherwise specified) usu. refers to the pH=2.5.[39]
Use
- Identify intestinal metaplasia in the stomach and esophagus -- goblets = blue.
Note:
- Esophageal submucosal glands - alcian blue positive.
Interpretation
- Blue = acidic mucins.[40]
Notes:
- Mucin stains:
Image
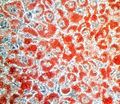
Barrett's type mucosa. Alcian blue stain. (WC/AFIP)
Barrett's type mucosa. Alcian blue stain. (WC/AFIP)
Sodium sulphate-alcian blue stain
- Sulfated alcian blue (abbreviated 'SAB) redirects here.
Use
- Identify amyloid.[41][42]
- Less specific than congo red but equally sensitive.
- Does not require polarized light.
Interpretation
- Green = amyloid.[41]
- Other things that are green: mast cells, mucoid degeneration, basophilic myofibre degeneration, califications.
- Yellow = background.
Image:
Movat's stain
Use
- Myxomatous degeneration of cardiac valves.
Components
Interpretation of Movat stain
- Black = nuclei and elastic fibers.
- Yellow = collagen and reticular fibers.
- Blue = mucin, ground substance.
- Red (intense) = fibrin.
- Red = muscle.
Reference: [44]
How to remember? A.: Primary colours (red, blue, yellow) + black.
Images
Cardiac amyloidosis - Movat stain. (WC/Nephron)
Cystic medial degeneration - Movat stain - low mag. (WC/Nephron)
Masson's trichrome stain
- Should not be confused with the Mallory trichrome stain.
- May be referred to as trichrome stain.
General
- Collagen vs. muscle.
Interpretation
- Black = nuclei.
- Red = muscle (smooth muscle actin).
- Baby blue = collagen.
Notes: [45]
Elastic trichrome stain
General:
- "Elastic trichrome" is one important variant of Masson's trichrome.
Interpretation - as above in Masson's trichrome - plus:
- Black = nuclei and elastin.
Mallory trichome stain
- Should not be confused with Masson trichrome stain.
- May be referred to as trichrome stain.
General
- Collagen vs. muscle.
- May be done with elastin.
Site
- Kidney Bx (to assess for fibrosis).
- Considered better than the Masson trichrome stain.
- Liver Bx (to assess for cirrhosis).
- Cardiovascular/lung (to see differentiate the layers of the arteries, and arteries from veins).
Interpretation
- Black = nuclei.
- Red = muscle (smooth muscle actin).
- Green = collagen.
Image
Cirrhosis. Mallory trichrome. (WC/Nephron)
Haematoxylin orcein phyloxin saffron stain
Interpretation
- Blue (haematoxylin) = nuclei.
- Black (orcein) = elastin.
- Red (phyloxin) = muscle.
- Yellow (saffron) = collagen.
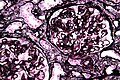
Jones stain
Use
- Visualize basement membrane in kidney biopsies.
- Especially useful for the diagnosis of membranous nephropathy (MN).
Interpretation
- Black = basement membrane.
- Blue = nuclei.
- Pink = other structures/background.
Notes:[48]
Images
MN demonstrated with a MPAS - very high mag. (WC/Nephron)
Hale's colloidal iron stain
Use
- Chromophobe renal cell carcinoma vs. renal oncocytoma - chromophobe renal cell carcinoma +ve.[49]
Notes:
Interpretation
- Blue (granular cytoplasmic) staining is positive.[7]
Images:
Notes:
- Often described as a "fastidious" (difficult/demanding) stain.[50]
- A few staff think this is a totally useless stain.[51]
- A variant exists known as the Muller and Mowry modification of Hale's colloidal iron stain (AKA Müller-Mowry stain).[52]
von Kossa stain
Use
- Look for calcium.
Interpretation
- Black = calcium.[7]
Toluidine blue stain
Use
- May be useful in kidney biopsies.[53][54]
- Stains mast cells, pneumocystis jirovecii.
Interpretation
- Dark blue - nuclei, mast cell granules (darker than nuclei).
- Light blue - cytoplasm.
- Red/magneta - cartilage. (???)
Refs: looks a bit sketchy[55], [56]
Image
PCP stained with toluidine blue. (WC)
www:
Romanowsky stain
- Occasionally spelled Romanowski.
- Many variants of this stain exist.
- Specimens are air-dried.
Interpretation:[57]
- Red - RBCs, eosinophil granules.
- Blue (basophilic) - lymphocyte cytoplasm.
- Purple - nuclear chromatin, neutrophil granules, platelets.
Field stain
- Variant of the Romanowsky stain for rapid processing.
- Tends to "blow-up" cell, i.e. cells are larger vis-a-vis Pap stain.
Diff-Quik
- Pronounced Diff-Quick.
- Proprietary variant of Romanowsky stain.[58]
Uses:
- Cytopathology.
- Helicobacter gastritis - organisms are dark blue against a light blue background.[59]
Wright stain
- A variant of the Romanowsky stain; popular in North American.
Use:
- Blood films.
May-Grünwald-Giemsa stain
- A variant of the Romanowsky stain; popular in Europe.
- Abbreviated MGG.
Use:
- Blood films.
- Cytopathology.
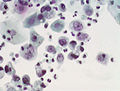
Papanicolaou stain
- Abbreviated Pap stain.
- Can be thought of as the H&E of cytopathology.
- It is a modified H&E stain.
- Specimens are fixed in ethanol.
- Good for seeing nuclear detail.
- Out-of-focus cytoplasm is translucent; allows one to focus overlapped cells in different planes.
Use
- Cytopathology.
Interpretation
- Blue/purple = nucleus.
- Green/pink = cytoplasm.
- Orange = keratin.
Image
Pap stain - urine cytology (WC)
Fontana-Masson stain
- AKA Masson-Fontana stain,[60] Fontana-Masson stain for melanin, melanin stain.
- A type of silver stain.
Stains:
- Melanin.
- "Argentaffin granules" of the digestive tract.
- Pigment deposition due to minocycline treatment.[61]
Use
- Stain for melanin.
- Displays melanin - whether it be in melanocytes, keratinocytes or melanophages.
- IHC stains, i.e. Melan A, SOX10 or MITF are preferable for displaying melanocytes.
- Used to differentiate brown pigments (lipofuscin, hemosiderin, melanin).[62]
- Used to document Minocycline type II drug induced pigment deposition
- Minocycline pigment, Type II will stain with both the Fontana-Masson stain AND the Perls iron stain.[63]
- Used in the differential diagnosis of hypomelanosis
- Idiopathic hypomelanosis will demonstrate ONLY loss of melanin with the Fontana-Masson stain - melanocytes will not be absent with the Melan A stain.
- Vitiligo will show loss of melanin AND loss of melanocytes with a Melan A stain.
Image:
Schmorl's stain
- Stains melanin.
- Similar to Fontana-Masson stain.
Notes:[64]
Martius scarlet blue stain
General
- Stains connective tissue and fibrin.[65]
- Abbreviated MSB.
Use:
- Look for fibrinoid necrosis in vasculitis.
Interpretation
- Muscle and fibrin - red.
- Nuclei = brown/black.
- Collagen - blue.
- Red blood cells - yellow.
Image:
Ref.:[66]
Picro-Mallory stain
General
- Find fibrin.
Interpretation[67]
- Fibrin = red.
- Erythrocytes = yellow.
- Connective tissue = blue.
Image:
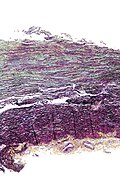
Verhoeff-van Gieson stain
- Verhoeff stain redirects here.
- AKA Elastic van Gieson stain, abbreviated EVG.
General
- Similar to Masson Trichrome & Verhoeff stain.[68]
Use:
- Examine large blood vessels.[69]
Interpretation
- Elastin = black.
- Collagen = bright red.
- Muscle = dull red.
Copper stain
General
- Used in liver biopsies.
- May be seen in Wilson's disease.
Note:
- Copper staining is a non-specific finding seen in many liver diseases; it is associated with impaired bile secretion.[70]
Interpretation
- Copper = red granules.
Images:
Shikata stain
General
- Used in medical liver biopsies - used to find copper.
Interpretation
Features:[74]
- Purple/brown = elastin fibres.
- Red = nuclei.
- Light purple = background
- ??? = Copper associated protein.
Gömöri Trichrome stain
- named after George Gömöri[75]
General
- Used in muscle biopsies - used to find abnormal mitochondrial deposits.
Interpretation
- Dark green = muscle fibers.
- Red = nuclei.
- Bright red = mitochondria, red blood cells.
Images:
See also
References
- ↑ LAE. 13 July 2010.
- ↑ Woods GL, Walker DH (July 1996). "Detection of infection or infectious agents by use of cytologic and histologic stains". Clin. Microbiol. Rev. 9 (3): 382-404. PMC 172900. PMID 8809467. http://cmr.asm.org/cgi/pmidlookup?view=long&pmid=8809467.
- ↑ Kovi J, Leifer C (July 1970). "Lipofuscin pigment accumulation in spontaneous mammary carcinoma of A/Jax mouse". J Natl Med Assoc 62 (4): 287–90. PMC 2611776. PMID 5463681. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2611776/pdf/jnma00512-0077.pdf.
- ↑ http://library.med.utah.edu/WebPath/HISTHTML/MANUALS/PAS.PDF
- ↑ Qizilbash, A.; Young-Pong, O. (Jun 1983). "Alpha 1 antitrypsin liver disease differential diagnosis of PAS-positive, diastase-resistant globules in liver cells.". Am J Clin Pathol 79 (6): 697-702. PMID 6189389.
- ↑ http://library.med.utah.edu/WebPath/HISTHTML/MANUALS/PASD.PDF
- ↑ 7.0 7.1 7.2 7.3 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 682. ISBN 978-0781765275.
- ↑ URL: http://library.med.utah.edu/WebPath/HISTHTML/MANUALS/FITES.PDF. Accessed on: 19 May 2011.
- ↑ URL: http://library.med.utah.edu/WebPath/LUNGHTML/LUNG024.html. Accessed on: 19 May 2011.
- ↑ Kehl, KS.; Cicirello, H.; Havens, PL. (Feb 1995). "Comparison of four different methods for detection of Cryptosporidium species.". J Clin Microbiol 33 (2): 416-8. PMID 7536216.
- ↑ Ignatius, R.; Lehmann, M.; Miksits, K.; Regnath, T.; Arvand, M.; Engelmann, E.; Futh, U.; Hahn, H. et al. (Feb 1997). "A new acid-fast trichrome stain for simultaneous detection of Cryptosporidium parvum and microsporidial species in stool specimens.". J Clin Microbiol 35 (2): 446-9. PMID 9003613.
- ↑ URL: http://www.ihcworld.com/_protocols/special_stains/congo_red_bennhold.htm. Accessed on: 26 January 2012.
- ↑ URL: http://library.med.utah.edu/WebPath/HISTHTML/MANUALS/CONGORED.PDF. Accessed on: 4 December 2010.
- ↑ St. Michael's Hospital - Stains Handout.
- ↑ URL: http://www.atsu.edu/faculty/chamberlain/Website/pnebact.htm. Accessed on: 7 May 2013.
- ↑ URL: http://meded.ucsd.edu/isp/1999/CAP/legion.html. Accessed on: 7 May 2013.
- ↑ URL: http://library.med.utah.edu/WebPath/HISTHTML/MANUALS/GRAM.PDF. Accessed on: 7 May 2013.
- ↑ URL: http://www.lpi.usra.edu/publications/slidesets/marslife/slide_27.html.
- ↑ MUN. 26 November 2010.
- ↑ http://library.med.utah.edu/WebPath/HISTHTML/MANUALS/LFB.PDF
- ↑ URL: http://library.med.utah.edu/WebPath/HISTHTML/STAINS/STAINS.html. Accessed on: April 6, 2009.
- ↑ MACKENZIE DH (March 1958). "Reticulin patterns in the diagnosis of carcinomas and sarcomas". Br. J. Cancer 12 (1): 14–9. PMC 2074006. PMID 13536209. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2074006/.
- ↑ http://library.med.utah.edu/WebPath/HISTHTML/MANUALS/RETIC.PDF
- ↑ Prussian blue stain. URL:[http://education.vetmed.vt.edu/curriculum/VM8054/labs/Lab2/Examples/exprussb.htm. Accessed on: 5 May 2010.
- ↑ Kluver-Barrera stain. URL:http://education.vetmed.vt.edu/curriculum/VM8054/labs/Lab2/Examples/exkluvbarr.htm. Accessed on: 5 May 2010.
- ↑ URL: http://www.merriam-webster.com/medical/warthin. Accessed on: 17 August 2010.
- ↑ URL: http://library.med.utah.edu/WebPath/HISTHTML/STAINS/STAINS.html. Accessed on: April 6, 2009.
- ↑ http://www.dako.co.uk/index/prod_search/prod_products.htm?productareaid=41&baseprodidver=A224462007
- ↑ http://library.med.utah.edu/WebPath/HISTHTML/STAINS/STAIN029.html
- ↑ DB. 4 August 2010.
- ↑ URL: http://www.mayomedicallaboratories.com/test-catalog/Overview/80327. Accessed on: 8 August 2010.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 455. ISBN 978-0781765275.
- ↑ URL: http://www.mondofacto.com/facts/dictionary?Dieterle%27s+stain. Accessed on: 4 August 2010.
- ↑ URL: http://www.mayomedicallaboratories.com/test-catalog/Overview/80327. Accessed on: 8 August 2010.
- ↑ http://library.med.utah.edu/WebPath/HISTHTML/MANUALS/BIELSCH.PDF
- ↑ Lefkowitch, Jay H. (2006). Anatomic Pathology Board Review (1st ed.). Saunders. pp. 681 (Q25). ISBN 978-1416025887.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 678. ISBN 978-0781765275.
- ↑ http://www.medschool.lsuhsc.edu/pathology/pathist/SURGPATH/special%20stains/assets/mucicarmine3.jpg
- ↑ URL: http://www.pathologyoutlines.com/topic/stainsalcianblue.html. Accessed on: 11 October 2012.
- ↑ URL: http://library.med.utah.edu/WebPath/HISTHTML/MANUALS/ALCIAN.PDF. Accessed on: 20 December 2011.
- ↑ 41.0 41.1 Pomerance, A.; Slavin, G.; McWatt, J. (Jan 1976). "Experience with the sodium sulphate-Alcian Blue stain for amyloid in cardiac pathology.". J Clin Pathol 29 (1): 22-6. PMID 55419.
- ↑ URL: http://www.polyrnd.com/products/reagent-assembly-kits/conventional/amyloid-stain---sulfated-alcian-blue-(sab).aspx. Accessed on: October 15, 2014.
- ↑ [1]
- ↑ 44.0 44.1 Modified Movat's Pentachrome Stain. University Penn Medicine. URL: http://www.med.upenn.edu/mcrc/histology_core/movat.shtml. Accessed on: January 29, 2009.
- ↑ ULR: http://library.med.utah.edu/WebPath/HISTHTML/MANUALS/MASSONS.PDF. Accessed on: 2 November 2011.
- ↑ Perry JR, Bilbao JM, Gray T (1992). "Fatal basilar vasculopathy complicating bacterial meningitis". Stroke 23 (8): 1175–8. PMID 1636194. Free Full Text.
- ↑ Jones, DB.. "Nephrotic glomerulonephritis.". Am J Pathol 33 (2): 313-29. PMC 1934622. PMID 13402889. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1934622/.
- ↑ URL: http://library.med.utah.edu/WebPath/HISTHTML/MANUALS/JONES.PDF. Accessed on: 19 May 2011.
- ↑ Tickoo SK, Amin MB, Zarbo RJ (April 1998). "Colloidal iron staining in renal epithelial neoplasms, including chromophobe renal cell carcinoma: emphasis on technique and patterns of staining". Am. J. Surg. Pathol. 22 (4): 419–24. PMID 9537468. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0147-5185&volume=22&issue=4&spage=419.
- ↑ URL: http://www.merriam-webster.com/dictionary/fastidious?show=0&t=1319550566. Accessed on: 25 October 2011.
- ↑ ALS. On several occasions in 2009.
- ↑ Mete, O.; Kilicaslan, I.; Gulluoglu, MG.; Uysal, V. (Dec 2005). "Can renal oncocytoma be differentiated from its renal mimics? The utility of anti-mitochondrial, caveolin 1, CD63 and cytokeratin 14 antibodies in the differential diagnosis.". Virchows Arch 447 (6): 938-46. doi:10.1007/s00428-005-0048-6. PMID 16133362.
- ↑ Fischer EG, Moore MJ, Lager DJ (October 2006). "Fabry disease: a morphologic study of 11 cases". Mod. Pathol. 19 (10): 1295–301. doi:10.1038/modpathol.3800634. PMID 16799480. http://www.nature.com/modpathol/journal/v19/n10/abs/3800634a.html.
- ↑ Nicholas, SB.; Basgen, JM.; Sinha, S. (2011). "Using stereologic techniques for podocyte counting in the mouse: shifting the paradigm.". Am J Nephrol 33 Suppl 1: 1-7. doi:10.1159/000327564. PMID 21659728.
- ↑ URL: http://www.molecularstation.com/protocol-links/articles/Toluidine-Blue-Stain-32.html. Accessed on: 17 March 2011.
- ↑ URL: http://www.dermnetnz.org/doctors/dermatopathology/stains.html. Accessed on: 17 March 2011.
- ↑ Horobin RW, Walter KJ (1987). "Understanding Romanowsky staining. I: The Romanowsky-Giemsa effect in blood smears". Histochemistry 86 (3): 331–6. PMID 2437082. http://www.springerlink.com/content/r81x25451m841866/.
- ↑ URL: http://www.ihcworld.com/_protocols/special_stains/diff_quick_ellis.htm. Accessed on: 4 January 2010.
- ↑ URL: http://www.ihcworld.com/_protocols/special_stains/diff_quick_ellis.htm. Accessed on: 30 August 2012.
- ↑ Gaitanis, G.; Chasapi, V.; Velegraki, A. (Aug 2005). "Novel application of the masson-fontana stain for demonstrating Malassezia species melanin-like pigment production in vitro and in clinical specimens.". J Clin Microbiol 43 (8): 4147-51. doi:10.1128/JCM.43.8.4147-4151.2005. PMID 16081962.
- ↑ Patterson, JW.; Wilson, B.; Wick, MR.; Heath, C. (Nov 2004). "Hyperpigmented scar due to minocycline therapy.". Cutis 74 (5): 293-8. PMID 15605966.
- ↑ URL: http://education.vetmed.vt.edu/curriculum/VM8054/labs/Lab2/Examples/exfontana.htm. Accessed on: 5 May 2010.
- ↑ Geria AN, Tajirian AL, Kihiczak G, Schwartz RA (2009). "Minocycline-induced skin pigmentation: an update". Acta Dermatovenerol Croat 17 (2): 123–6. PMID 19595269.
- ↑ URL: http://library.med.utah.edu/WebPath/HISTHTML/STAINS/STAINS.html. Accessed on: 5 May 2010.
- ↑ URL: http://www.bris.ac.uk/vetpath/cpl/msb.html. Accessed on: 26 November 2010.
- ↑ URL: http://www.bris.ac.uk/vetpath/cpl/msb.html. Accessed on: 26 November 2010.
- ↑ "Picro-Mallory for Fibrin – Long Version". http://stainsfile.info/StainsFile/stain/fibrin/picro-mallory-1.htm. Retrieved 17 January 2011.
- ↑ URL: http://education.vetmed.vt.edu/Curriculum/VM8054/Labs/Lab2/Examples/exvrmass.htm. Accessed on: 3 January 2011.
- ↑ URL: http://education.vetmed.vt.edu/Curriculum/VM8054/Labs/Lab2/Examples/exvvg.htm. Accessed on: 3 January 2011.
- ↑ Miyamura H, Nakanuma Y, Kono N (December 1988). "Survey of copper granules in liver biopsy specimens from various liver abnormalities other than Wilson's disease and biliary diseases". Gastroenterol. Jpn. 23 (6): 633–8. PMID 2464523.
- ↑ URL: http://www.naika.or.jp/im2/42/10/14c.aspx. Accessed on: 24 January 2011.
- ↑ http://www.mayomedicallaboratories.com/test-catalog/Overview/9836. Accessed on: 24 January 2011.
- ↑ URL: http://informahealthcare.com/doi/abs/10.3109/00313027709085239?journalCode=pat. Accessed on: 24 January 2011.
- ↑ URL: http://www.nottingham.ac.uk/pathology/protocols/shikata.html. Accessed on: 24 January 2011.
- ↑ GOMORI, G. - A rapid one-step trichrome stain. Am. J. Clin. Path. 20: 661-664, 1950.
External links
- Procedure manuals - med.utah.edu.
- Special stains (introduction) - med.utah.edu.
- Stains - histology-world.com.