Difference between revisions of "Endometrium"
| Line 283: | Line 283: | ||
| [[disordered proliferative phase]], [[simple endometrial hyperplasia]], [[complex endometrial hyperplasia]], early [[secretory phase endometrium]] | | [[disordered proliferative phase]], [[simple endometrial hyperplasia]], [[complex endometrial hyperplasia]], early [[secretory phase endometrium]] | ||
| normal | | normal | ||
| Image | | [[Image:Proliferative endometrium - very high mag.jpg|thumb|150px|center|Proliferative endometrium. (WC)]] | ||
|- | |- | ||
| [[Secretory phase endometrium]] | | [[Secretory phase endometrium]] | ||
| Line 311: | Line 311: | ||
| [[secretory phase endometrium]], [[endometrial hyperplasia with secretory changes]] | | [[secretory phase endometrium]], [[endometrial hyperplasia with secretory changes]] | ||
| Other | | Other | ||
| [[Image:Endometrium ocp use3.jpg|thumb|150px|Endometrium with OCP changes]] | | [[Image:Endometrium ocp use3.jpg|thumb|150px|center|Endometrium with OCP changes. (WC)]] | ||
|- <!-- | |- <!-- | ||
| Diagnosis | | Diagnosis | ||
Revision as of 03:48, 16 November 2013
The endometrium is typically biopsied because of abnormal bleeding. Endometrial hyperplasia and endometrial carcinoma are dealt with in separate articles. An overview of gynecologic pathology is in the gynecologic pathology article.
Indications for endometrial biopsy
Abnormal bleeding:
- Abnormal uterine bleeding (AUB).
- Dysfunctional uterine bleeding, abbreviated DUB, is diagnosed if other causes of bleeding are excluded.
- DUB may get a D&C if they fail medical management.[1]
- Post-menopausal bleeding.
Other indications:[2]
- Products of conception - dealt with in a separate article.
- Dating of endometrium - infertility work-up.
Normal microscopic findings
Endometrium - consists of:
- Epithelium (endometrial glands).
- Stroma (endometrial stroma).
In endometrial biopsies:
- Endocervical glands are commonly seen, as is endocervical mucous.
- This is 'cause the gynecologist scrapes some off on the way in or out.
Glandular telescoping
- Considered an artifact of tissue processing, i.e. normal.[3]
Image:
Compression artifact
- Gland moulding.
- Tearing of tissue around the compressed glands - key feature.
- Usually at the edge of a tissue fragment.
DDx:
Image:
Endocervical epithelium versus endometrial epithelium
Table
| Feature | Endometrial | Endocervical | Tubal metaplasia |
|---|---|---|---|
| Cytoplasmic staining | usu. hyperchromatic +/-vacuoles | clear or light eosinophilic | hyperchromatic |
| Nuclear-to-cytoplasm ratio | moderate to high (1:2) | low (often 1:3) | high (1:1) |
| Surface features | villi | ||
| Associated stroma | cellular, hyperchromatic | inflamed, less cellular | variable |
List
Endocervical:
- Less hyperchromatic.
- Nuclei round & small.
- Cell borders usually well-defined.
Endometrial:
- More hyperchromatic.
- Nuclei columnar.
Images
Metaplasias of the endometrium
The big table of metaplasias - adapted from Nicolae et al.:[4]
| Metaplasia | Subtypes | Microscopic | Notes | Risk of malignancy |
|---|---|---|---|---|
| Morules | - | nearly always | ||
| Ciliary | - | ciliated cells | usu. lumped together with tubal, unopposed estrogen, endometriosis | frequent - endometrial hyperplasia (complex and simple), adenocarcinoma |
| Tubal | complex, simple | ciliated cells, secretory cell, intercallary cells | usu. lumped together with ciliary, unopposed estrogen, seen in endometriosis | frequent (complex only) - endometrial hyperplasia (complex and simple), adenocarcinoma |
| Mucinous | complex, simple | frequent (complex only) | ||
| Squamous | - | rare | ||
| Papillary syncytial change (surface) | - | rare | ||
| Eosinophilic, oxyphilic, oncocytic | not known | |||
| Clear cell (secretory) | - | not reported | ||
| Stromal metaplasia | osseous, cartilaginous, adipose, smooth muscle, myoid, sex-cord like | not reported |
Tamoxifen effects
Inadequate endometrial biopsy
- Endometrial biopsies often have scant tissue.
- This is normal in post-menopausal women.
- Ideally, the biopsy should have some endometrial stroma.
- Without stroma it is not possible to assess the gland-to-stroma ratio.
Sign out
No stroma
ENDOMETRIUM, BIOPSY: - VERY SCANT STRIPPED NON-PROLIFERATIVE COLUMNAR EPITHELIUM, PROBABLY FROM THE LOWER UTERINE SEGMENT. - NO DEFINITE ENDOMETRIAL STROMA, SEE COMMENT. - STRIPPED ENDOCERVICAL EPITHELIUM AND ENODOCERVICAL MUCOSA WITHIN NORMAL LIMITS. - MUCOUS. COMMENT: A re-biopsy should be considered within the clinical context.
ENDOMETRIUM, BIOPSY: - VERY SCANT STRIPPED EPITHELIUM PROBABLY FROM THE LOWER UTERINE SEGMENT. - NO DEFINITE ENDOMETRIAL STROMA. - SCANT STRIPPED ENDOCERVICAL EPITHELIUM WITHIN NORMAL LIMITS. - SMALL FRAGMENTS OF DETACHED BENIGN SQUAMOUS EPITHELIUM. COMMENT: A re-biopsy should be considered within the clinical context.
ENDOMETRIUM, BIOPSY: - FRAGMENTS OF DETACHED SQUAMOUS EPITHELIUM AND DETACHED NON-PROLIFERATIVE ENDOMETRIAL GLANDS. - ASSESSMENT LIMITED AS VERY SCANT ENDOMETRIAL STROMA IS PRESENT.
ENDOMETRIUM, BIOPSY: - ENDOMETRIUM: STRIPS OF EPITHELIUM, NON-PROLIFERATIVE. - ENDOCERVIX: SCANT BENIGN EPITHELIUM. - EXOCERVIX: SCANT BENIGN EPITHELIUM. - OTHER: TUBAL METAPLASIA.
ENDOMETRIUM, BIOPSY: - STRIPS OF NON-PROLIFERATIVE ENDOMETRIUM. - SCANT BENIGN ENDOCERVICAL EPITHELIUM. - SCANT BENIGN SQUAMOUS EPITHELIUM. - TUBAL METAPLASIA.
ENDOMETRIUM, BIOPSY: - STRIPS OF BENIGN ENDOMETRIAL EPITHELIUM/TUBAL METAPLASIA, NON-PROLIFERATIVE. - SCANT BENIGN ENDOCERVICAL EPITHELIUM. - RARE SQUAMOUS METAPLASTIC CELLS.
Proliferative without definite stroma
ENDOMETRIUM, BIOPSY: - FRAGMENTS OF DETACHED SQUAMOUS EPITHELIUM, ENDOCERVICAL EPITHELIUM AND FOCALLY PROLIFERATIVE ENDOMETRIAL GLANDS. - ASSESSMENT LIMITED AS NO DEFINITE ENDOMETRIAL STROMA IS PRESENT.
No endometrium
ENDOMETRIUM, BIOPSY: - SPECIMEN INADEQUATE; NO ENDOMETRIUM IDENTIFIED. - ONE VERY TINY FRAGMENT OF ENDOCERVICAL MUCOSA WITHOUT APPARENT PATHOLOGY.
ENDOMETRIUM, BIOPSY: - ENDOCERVICAL MUCOSA AND STRIPPED ENDOCERVICAL EPITHELIUM WITHIN NORMAL LIMITS. - MICROGLANDULAR HYPERPLASIA AND FOCAL SQUAMOUS METAPLASIA. - NO DEFINITE ENDOMETRIUM IDENTIFIED, SUGGEST RE-BIOPSY.
No tissue
ENDOMETRIUM, BIOPSY: - NO TISSUE IDENTIFIED ON MICROSCOPY.
Overview
A simple approach
Low power
- Decide whether you are looking at endometrium.
- Is the gland-to-stroma ratio normal?
- 1:3 is normal.
- If the gland-to-stroma ratio is increased... think complex endometrial hyperplasia.
- If the glands are fused to one another or cribriform... think endometrial carcinoma.
- Glands round?
- Round is normal.
- Irregular - may be seen in secretory phase endometrium, menses, disordered proliferative endometrium (focal), simple endometrial hyperplasia (diffuse).
- Glands pseudostratified?
- Pseudostratified glands are normal in the proliferative phase endometrium, hyperplasias, malignancy.
- Balls of cells?
- Blue - likely menstrual (stromal condensation).
- Pink - consider leiomyoma, squamous morules (associated with endometrial hyperplasia, endometrioid endometrial carcinoma, may be benign).
High power
- Mitoses present in the glands?
- Present in the proliferative phase, hyperplasias, malignancies.
- Mitoses present in the stroma?
- Present in the proliferative phase, hyperplasias, malignancies.
- Mucous present in the glands?
- Present in the secretory phase.
- Inflammatory cells present?
- Some are normal during menses.
Tabular summary
| Diagnosis | Key feature (low power) | Additional features | DDx | Other | Image |
|---|---|---|---|---|---|
| Proliferative phase endometrium | round spaced pseudostratified glands | mitoses in glands and stroma | disordered proliferative phase, simple endometrial hyperplasia, complex endometrial hyperplasia, early secretory phase endometrium | normal | |
| Secretory phase endometrium | irregular glands with secretions or simple glands with vacuoles | decidual changes (nucleus central, eosinophilic cytoplasm, well-defined cell borders) | endometrial hyperplasia with secretory changes, late proliferative phase endometrium | normal | Image |
| Menstrual endometrium | stromal condensation | nonproliferative glands, stromal/epithelial neutrophils, glandular cell apoptosis | disordered proliferative phase | normal | Image |
| Benign endometrial polyp | fibrous stroma, muscular blood vessels | polypoid shape (epithelium on 3 sides), +/-gland dilation | disordered proliferative phase, simple endometrial hyperplasia | Other | Image |
| Endometrium with changes due to exogenous hormones | decidualized stroma (nucleus central, eosinophilic cytoplasm, well-defined cell borders) | inactive glands (round/ovoid glands, simple cuboidal epithelium, no mitoses) | secretory phase endometrium, endometrial hyperplasia with secretory changes | Other |
Normal endometrium
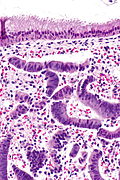
Proliferative phase endometrium
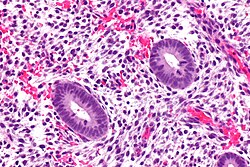
Secretory phase endometrium
Menstrual endometrium
General
- Technically part of the proliferative phase or follicular phase.
Microscopic
Features:
- Proliferative endometrium (mitoses).[5]
- Apoptotic cells common.[6]
- Tightly packed cellular balls of stromal cells with nuclear moulding.
- Known as "blue balls".
- Tightly packed cellular stromal cells known as "stromal condensation".
- Inflammation, especially abundant neutrophils.
DDx:
- Small cell carcinoma.
- Anovulatory endometrium - less neutrophils.
Images
www:
Sign out
ENDOMETRIUM, BIOPSY: - CONSISTENT WITH MENSTRUAL ENDOMETRIUM: -- STRIPPED WEAKLY PROLIFERATIVE ENDOMETRIAL GLANDS. -- BALLS OF CONDENSED ENDOMETRIAL STROMA. -- ABUNDANT NEUTROPHILS AND BLOOD.
ENDOMETRIUM, BIOPSY: - CONSISTENT WITH MENSTRUAL PHASE ENDOMETRIUM: -- WEAKLY PROLIFERATIVE ENDOMETRIAL GLANDS WITH NEUTROPHILS AND APOPTOSIS. -- BALLS OF CONDENSED ENDOMETRIAL STROMA. -- BLOOD.
ENDOMETRIUM, BIOPSY: - VERY WEAKLY PROLIFERATIVE ENDOMETRIAL GLANDS WITH NEUTROPHILS AND APOPTOSIS. - BALLS OF CONDENSED ENDOMETRIAL STROMA AND BLOOD. - NEGATIVE FOR HYPERPLASIA AND NEGATIVE FOR MALIGNANCY.
Late menses
ENDOMETRIUM, ASPIRATION: - ENDOMETRIAL GLANDS WITH APOPTOTIC CELLS, INFILTRATING NEUTROPHILS, AND GLANDULAR PROLIFERATIVE ACTIVITY. - BALLS OF CONDENSED ENDOMETRIAL STROMA. - SCANT STRIPPED ENDOCERVICAL EPITHELIUM WITHIN NORMAL LIMITS. - NEGATIVE FOR HYPERPLASIA. COMMENT: The findings are most in keeping with late menstrual endometrium.
Specific entities/abnormalities
Arias-Stella reaction
- Benign atypical endometrial changes associated with chorionic tissue -- may be seen in a completely normal pregnancy and misdiagnosed as a malignancy.[7]
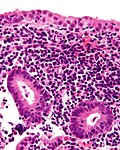
Endometritis
General
- Usually post-delivery or post-instrumentation, e.g. previous biopsy.
- May be spontaneous, e.g. tuberculous endometritis.
Microscopic
Acute endometritis
Features:
- Neutrophils clusters (>5 PMNs) in the:
- Endometrial stroma.
- Within uterine glands.
Notes:
- Neutrophils are normal in the context of menses.
Image:
Chronic endometritis
Features:[8]
- Plasma cells with in the endometrial stroma - key feature.
- Usually superficial/close to the luminal aspect.
- Lymphocytic infiltrate - usually marked.
- May form lymphoid aggregates - low power finding.
- +/-Eosinophils - presence should prompt a search for plasma cells.[9]
Other findings:[8]
- +/-Necrosis.
- Edema - common.
- Hemorrhage.
Notes:
- One plasma cell is not enough to call it.
DDx:
- Mentrual endometrium - endometrial stromal condensation.
Images
www:
- Chronic endometritis (webpathology.com).
- Chronic endometritis (webpathology.com).
- Tuberculous endometritis (webpathology.com).
Sign out
ENDOMETRIUM, BIOPSY: - CHRONIC ENDOMETRITIS.
Not definite endometritis
ENDOMETRIUM, ASPIRATION: - PROLIFERATIVE PHASE ENDOMETRIUM WITH A MILD LYMPHOCYTIC INFILTRATE AND VERY RARE PLASMA CELLS, SEE COMMENT. - NEGATIVE FOR HYPERPLASIA. COMMENT: The lymphocytic infiltrate and plasma cells raise the possibility of a mild chronic endometritis; clinical correlation is suggested.
Nonspecific lymphocytic infiltrate
If not more than one plasma cell is apparent after searching.
ENDOMETRIUM, ASPIRATION: - PROLIFERATIVE PHASE ENDOMETRIUM WITH A MILD LYMPHOCYTIC INFILTRATE. - SMALL FRAGMENT OF ENDOCERVICAL MUCOSA WITHIN NORMAL LIMITS. - NEGATIVE FOR HYPERPLASIA.
Micro
The section show proliferative endometrium with a normal gland-to-stroma ratio. Mitotic activity is seen in the glands and stroma. No cytologic atypia is apparent. A mild nonspecific lymphocytic infiltrate is present.
No lymphoid aggregates are apparent. No eosinophils are apparent. No significant number of plasma cells is apparent.
Benign endometrial polyp
Anovulatory endometrium
General
- May be used as a synonym for disordered proliferative phase.[10]
Microscopic
Features:
- Shedding:
- Stromal condensation.
- Apoptotic endometrial epithelium.
- Nonproliferative glands.
DDx:
- Disordered proliferative phase.
- Simple endometrial hyperplasia.
- Menstrual endometrium - should have mitoses,[5] abundant PMNs.
Sign out
ENDOMETRIUM, CURETTAGE: - NON-PROLIFERATIVE ENDOMETRIUM WITH SMALL ROUND GLANDS AND SHEDDING, SEE COMMENT. - BENIGN ENDOCERVICAL MUCOSA. - NEGATIVE FOR HYPERPLASIA. - NEGATIVE FOR MALIGNANCY. COMMENT: The changes are suggestive of anovulatory bleeding.
ENDOMETRIUM, BIOPSY: - BENIGN ENDOCERVICAL POLYP WITH ACUTE AND CHRONIC INFLAMMATION, AND EVIDENCE SUGGESTIVE OF EROSIONS (SIDEROPHAGES, INCREASED BLOOD VESSEL DENSITY). - SMALL NONPROLIFERATIVE ENDOMETRIAL GLANDS WITH RARE NEUTROPHILS AND RARE APOPTOTIC CELLS, WITH BALLS OF CONDENSED ENDOMETRIAL STROMA, SEE COMMENT. - NEGATIVE FOR ENDOMETRIAL HYPERPLASIA AND NEGATIVE FOR DYSPLASIA. COMMENT: The changes are suggestive of anovulatory bleeding.
Disordered proliferative endometrium
- Abbreviated DPE.
- AKA endometrium with disordered proliferative phase.
- AKA disordered proliferative phase.
General
- Association: anovulation.
- Benign - can be grouped with normal.[11]
Treatment:
Image:
Microscopic
Features:[13]
- Proliferative type endometrium with:
- Cystic dilation of glands focally that do not have (glandular) secretions - key feature.
- Glands >2x normal size - usually 3-4x normal.
- Irregular shape, e.g. gland contour has inflection points.
- Greater than fours glands involved (dilated).
- Cystic dilation of glands focally that do not have (glandular) secretions - key feature.
- +/-Stromal condensation -- balls of stromal tissue, aka "blue balls" (due to breakdown of endometrium).
Notes:
- Dilated glands often have tubal metaplasia.[citation needed]
- Eosinophilic syncytial metaplasia - common.
- Features: abundant eosinophilic cytoplasm, mild nuclear atypia +/-loss of nuclear stratification, no mitoses).
DDx:
- Proliferative phase endometrium.
- Glands: straight, tubular, tall pseudostratified columnar cells, mitotic figures, no vacuolation, no mucus secretion, abundant mitoses.
- Stroma: cellular, stroma (spindle cells), mitoses.
- Simple endometrial hyperplasia without atypia - architectural atypia diffuse.
- Benign endometrial polyp.
Images
www:
Sign out
ENDOMETRIUM, BIOPSY: - DISORDERED PROLIFERATIVE ENDOMETRIUM.
With endocervix
ENDOMETRIUM, BIOPSY: - DISORDERED PROLIFERATIVE ENDOMETRIUM. - BENIGN ENDOCERVICAL MUCOSA.
Waffle a bit
ENDOMETRIUM, BIOPSY: - COMPATIBLE WITH DISORDERED PROLIFERATIVE ENDOMETRIUM (FRAGMENTS OF PROLIFERATIVE ENDOMETRIUM WITH EVIDENCE OF SHEDDING AND VERY RARE GLAND DILATION). - VERY SCANT STRIPPED ENDOCERVICAL EPITHELIUM WITHOUT APPARENT PATHOLOGY. - NEGATIVE FOR ENDOMETRIAL HYPERPLASIA. - NEGATIVE FOR MALIGNANCY.
Micro
The sections show a well-sampled endometrium. Mitotic figures are identified within the glands and stroma. Irregular, moderately enlarged glands are seen (only) in one of several fragments; most of the endometrial glands are round, regular and small.
No stromal condensation is apparent. No secretions are in the glands.
There are no back-to-back glands. No nuclear atypia is apparent. No thick-walled blood vessels are apparent.
Endometrium with changes due to exogenous hormones
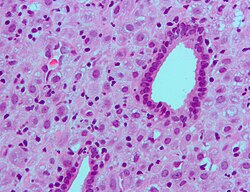
Atrophic endometrium
- Inactive endometrium redirect here.
General
- Endometrium of normal postmenopausal women.
- Menopause typically happens at around 50 years old.
- Very common diagnosis.
- Atrophy may be associated with bleeding and therefore biopsied to rule-out hyperplasia and malignancy.
Gross
- Thin endometrium.
Microscopic
Features:
- Glands - small columnar cells:
- Moderate quantity of eosinophilic cytoplasm.
- Ovoid (palisaded) nuclei +/- nuclear pseudostratification.[3]
- Eosinophilic cytoplasm.
- No mitoses.
- Architecture:
- +/-Cystic dilation.
Notes:
- If a woman is truly postmenopausal, mitoses in the glandular epithelium is pathologic until demonstrated otherwise.
- The exceptions are benign endometrial polyp, uterine prolapse, and possibly inflammation (e.g. the person has had several biopsy attempts and was seeded with pathogens).
DDx:
- Proliferative phase endometrium - esp. if there is pseudostratification.
- Serous carcinoma of the endometrium.
Images:
Sign out
ENDOMETRIUM, BIOPSY: - NON-PROLIFERATIVE ENDOMETRIUM. - BENIGN SQUAMOUS EPITHELIUM WITH METAPLASTIC CHANGE. - SCANT ENDOCERVICAL MUCOSA WITH REACTIVE CHANGES.
ENDOMETRIUM, BIOPSY: - NON-PROLIFERATIVE ENDOMETRIUM. - BENIGN STRIPPED ENDOCERVICAL EPITHELIUM. - NEGATIVE FOR HYPERPLASIA AND NEGATIVE FOR MALIGNANCY.
Micro
The sections show small fragments of endometrium. The gland-to-stroma ratio is normal. The glands are small and round, and have a pseudostratified epithelium.
Mitotic figures are not identified within the glands or stroma. No stromal condensation is apparent. No secretions are in the glands. No nuclear atypia is apparent.
Scant benign endocervical tissue (stripped epithelium and mucosa) is present.
Limited stroma
ENDOMETRIUM, BIOPSY: - STRIPPED NONPROLIFERATIVE ENDOMETRIAL EPITHELIUM; NO APPRECIABLE STROMA PRESENT. - SCANT ENDOCERVICAL EPITHELIUM WITHIN NORMAL LIMITS. - MINUTE FRAGMENTS OF SQUAMOUS EPITHELIUM WITHOUT APPARENT PATHOLOGY.
ENDOMETRIUM, BIOPSY: - SCANT STRIPPED NON-PROLIFERATIVE ENDOMETRIAL EPITHELIUM. - VERY SMALL FRAGMENT OF ENDOMETRIAL STROMA. - TUBAL METAPLASTIC EPITHELIUM.
ENDOMETRIUM, ASPIRATION: - SMALL FRAGMENTS OF NONPROLIFERATIVE ENDOMETRIAL EPITHELIUM ATTACHED TO A VERY SMALL AMOUNT OF STROMA. - MINUTE BENIGN FRAGMENT OF SQUAMOUS EPITHELIUM. - MUCOUS AND INFLAMMATORY CELLS. COMMENT: The sample is scant given the history of 'thickened endometrium'.
Micro
The sections show stripped endometrial epithelium and stripped tubal-type epithelium. No mitotic activity is identified. No nuclear atypia is apparent. A small fragment of definite endometrial stroma is present. The gland-to-stroma ratio cannot be assessed due to the limited stroma.
Endometrial hyperplasia
Can be thought of as a precursor lesion for endometrial carcinoma.
It comes in two main flavours:
- Simple.
- Complex.
Each flavour may or may not have nuclear atypia.
Endometrial carcinoma
Endometrial cancer is the most common gynecologic malignancy (in the USA).[15]
See also
References
- ↑ URL: http://emedicine.medscape.com/article/257007-treatment. Accessed on: 15 July 2010.
- ↑ Mazur, Michael T.; Kurman, Robert J. (2005). Diagnosis of Endometrial Biopsies and Curettings: A Practical Approach (2nd ed.). Springer. pp. 1. ISBN 978-0387986159.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 McCluggage, WG. (Aug 2006). "My approach to the interpretation of endometrial biopsies and curettings.". J Clin Pathol 59 (8): 801-12. doi:10.1136/jcp.2005.029702. PMID 16873562. Cite error: Invalid
<ref>tag; name "pmid16873562" defined multiple times with different content - ↑ Nicolae, A.; Preda, O.; Nogales, FF. (Feb 2011). "Endometrial metaplasias and reactive changes: a spectrum of altered differentiation.". J Clin Pathol 64 (2): 97-106. doi:10.1136/jcp.2010.085555. PMID 21126963.
- ↑ 5.0 5.1 Tadrous, Paul.J. Diagnostic Criteria Handbook in Histopathology: A Surgical Pathology Vade Mecum (1st ed.). Wiley. pp. 237. ISBN 978-0470519035.
- ↑ Spencer, SJ.; Cataldo, NA.; Jaffe, RB. (May 1996). "Apoptosis in the human female reproductive tract.". Obstet Gynecol Surv 51 (5): 314-23. PMID 8744416.
- ↑ Arias-Stella, J. (Jan 2002). "The Arias-Stella reaction: facts and fancies four decades after.". Adv Anat Pathol 9 (1): 12-23. PMID 11756756.
- ↑ 8.0 8.1 Tawfik, O.; Venuti, S.; Brown, S.; Collins, J. (1996). "Immunohistochemical characterization of leukocytic subpopulations in chronic endometritis.". Infect Dis Obstet Gynecol 4 (5): 287-93. doi:10.1155/S1064744996000555. PMC 2364507. PMID 18476109. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2364507/.
- ↑ Adegboyega, PA.; Pei, Y.; McLarty, J. (Jan 2010). "Relationship between eosinophils and chronic endometritis.". Hum Pathol 41 (1): 33-7. doi:10.1016/j.humpath.2009.07.008. PMID 19801162.
- ↑ URL: http://www.surgpath4u.com/caseviewer.php?case_no=382. Accessed on: 9 May 2013.
- ↑ Sherman, ME.; Ronnett, BM.; Ioffe, OB.; Richesson, DA.; Rush, BB.; Glass, AG.; Chatterjee, N.; Duggan, MA. et al. (Jul 2008). "Reproducibility of biopsy diagnoses of endometrial hyperplasia: evidence supporting a simplified classification.". Int J Gynecol Pathol 27 (3): 318-25. doi:10.1097/PGP.0b013e3181659167. PMID 18580308.
- ↑ 12.0 12.1 Ely, JW.; Kennedy, CM.; Clark, EC.; Bowdler, NC.. "Abnormal uterine bleeding: a management algorithm.". J Am Board Fam Med 19 (6): 590-602. PMID 17090792. http://www.jabfm.org/content/19/6/590.full.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 1080 and 1082. ISBN 0-7216-0187-1.
- ↑ URL: http://www.glowm.com/index.html?p=glowm.cml/section_view&articleid=235. Accessed on: 11 December 2012.
- ↑ Lu KH (April 2009). "Management of early-stage endometrial cancer". Semin. Oncol. 36 (2): 137–44. doi:10.1053/j.seminoncol.2008.12.005. PMID 19332248.