Difference between revisions of "Urothelial carcinoma"
| (9 intermediate revisions by 2 users not shown) | |||
| Line 7: | Line 7: | ||
| Micro = | | Micro = | ||
| Subtypes = [[microcystic urothelial carcinoma|microcystic]], [[micropapillary urothelial carcinoma|micropapillary]], glandular, inverted (growth pattern), [[nested urothelial carcinoma|nested]], papillary (dealt with separately in ''[[high-grade papillary urothelial carcinoma]]'' and ''[[low-grade papillary urothelial carcinoma]]''), [[plasmacytoid urothelial carcinoma]], others | | Subtypes = [[microcystic urothelial carcinoma|microcystic]], [[micropapillary urothelial carcinoma|micropapillary]], glandular, inverted (growth pattern), [[nested urothelial carcinoma|nested]], papillary (dealt with separately in ''[[high-grade papillary urothelial carcinoma]]'' and ''[[low-grade papillary urothelial carcinoma]]''), [[plasmacytoid urothelial carcinoma]], others | ||
| LMDDx = [[urothelial carcinoma in situ]], metastatic carcinoma ([[prostate carcinoma]], [[colorectal carcinoma]]), [[inverted urothelial papilloma]] (for UCC with inverted growth pattern) | | LMDDx = [[urothelial carcinoma in situ]], metastatic carcinoma ([[prostate carcinoma]], [[colorectal carcinoma]]), [[inverted urothelial papilloma]] (for UCC with inverted growth pattern), [[epithelioid angiosarcoma]] | ||
| Stains = | | Stains = | ||
| IHC = [[GATA3]] +ve, p63 +ve, CK5/6 +ve, CK34betaE12 +ve, PSA -ve | | IHC = [[GATA3]] +ve, p63 +ve, CK5/6 +ve, CK34betaE12 +ve, PSA -ve | ||
| EM = | | EM = | ||
| Molecular = not used for diagnosis; typically: 9p deletions, 17p deletions | | Molecular = not used for diagnosis; typically: 9p deletions, 17p deletions; can be [[Classification of urothelial carcinoma by immunohistochemistry|subclassified with IHC]]; [[FGFR2/FGFR3]] mutations | ||
| IF = | | IF = | ||
| Gross = | | Gross = | ||
| Grossing = [[radical cystectomy grossing]], [[cystoprostatectomy grossing]], [[nephroureterectomy grossing]] | | Grossing = [[transurethral resection of bladder tumour grossing]], [[radical cystectomy grossing]], [[cystoprostatectomy grossing]], [[nephroureterectomy grossing]] | ||
| Staging = [[bladder cancer staging]] | | Staging = [[bladder cancer staging]] | ||
| Site = [[urothelium]] - [[ureter]], [[urinary bladder]], proximal [[urethra]] (see [[urothelial carcinoma of the urethra]], renal pelvis | | Site = [[urothelium]] - [[ureter]], [[urinary bladder]], proximal [[urethra]] (see [[urothelial carcinoma of the urethra]], renal pelvis | ||
| Line 79: | Line 79: | ||
*[[Low-grade papillary urothelial carcinoma]]. | *[[Low-grade papillary urothelial carcinoma]]. | ||
*[[Prostate carcinoma]] - may have pseudopapillae<ref name=pmid24503758>{{cite journal |author=Gordetsky J, Epstein JI |title=Pseudopapillary features in prostatic adenocarcinoma mimicking urothelial carcinoma: a diagnostic pitfall |journal=Am. J. Surg. Pathol. |volume=38 |issue=7 |pages=941–5 |year=2014 |month=July |pmid=24503758 |doi=10.1097/PAS.0000000000000178 |url=}}</ref> - see ''[[urothelial carcinoma-like prostatic carcinoma]]''. | *[[Prostate carcinoma]] - may have pseudopapillae<ref name=pmid24503758>{{cite journal |author=Gordetsky J, Epstein JI |title=Pseudopapillary features in prostatic adenocarcinoma mimicking urothelial carcinoma: a diagnostic pitfall |journal=Am. J. Surg. Pathol. |volume=38 |issue=7 |pages=941–5 |year=2014 |month=July |pmid=24503758 |doi=10.1097/PAS.0000000000000178 |url=}}</ref> - see ''[[urothelial carcinoma-like prostatic carcinoma]]''. | ||
*[[Epithelioid angiosarcoma]] - have intracytoplasmic lumens and interspersed red blood cells, usually have a history radiation treatment.<ref name=pmid25929352>{{cite journal |authors=Matoso A, Epstein JI |title=Epithelioid Angiosarcoma of the Bladder: A Series of 9 Cases |journal=Am J Surg Pathol |volume=39 |issue=10 |pages=1377–82 |date=October 2015 |pmid=25929352 |doi=10.1097/PAS.0000000000000444 |url=}}</ref> | |||
==Staging== | ==Staging== | ||
| Line 105: | Line 106: | ||
www: | www: | ||
*[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3282447/figure/F2/ Several images of NUCC (nih.gov)].<ref name=pmid22355497>{{Cite journal | last1 = Terada | first1 = T. | title = Nested variant of urothelial carcinoma of the urinary bladder. | journal = Rare Tumors | volume = 3 | issue = 4 | pages = e42 | month = Oct | year = 2011 | doi = 10.4081/rt.2011.e42 | PMID = 22355497 | PMC = 3282447 }}</ref> | *[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3282447/figure/F2/ Several images of NUCC (nih.gov)].<ref name=pmid22355497>{{Cite journal | last1 = Terada | first1 = T. | title = Nested variant of urothelial carcinoma of the urinary bladder. | journal = Rare Tumors | volume = 3 | issue = 4 | pages = e42 | month = Oct | year = 2011 | doi = 10.4081/rt.2011.e42 | PMID = 22355497 | PMC = 3282447 }}</ref> | ||
[[File:5 410253052 sl 1.png| High grade urothelial carcinoma]] | |||
[[File:5 410253052 sl 2.png| High grade urothelial carcinoma]] | |||
[[File:5 410253052 sl 3.png| High grade urothelial carcinoma]] | |||
[[File:5 410253052 sl 4.png| High grade urothelial carcinoma]] | |||
[[File:5 410253052 sl 5.png| High grade urothelial carcinoma]] | |||
[[File:5 410253052 sl 6.png| High grade urothelial carcinoma]] | |||
[[File:5 410253052 sl 7.png| High grade urothelial carcinoma]] | |||
[[File:5 410253052 sl 8.png| High grade urothelial carcinoma]]<br> | |||
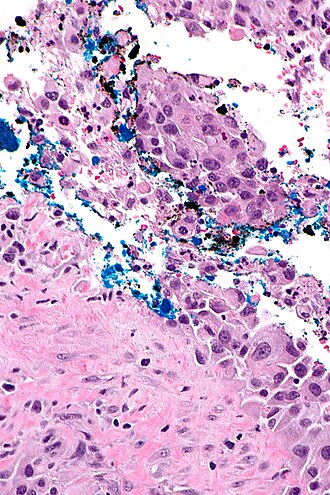
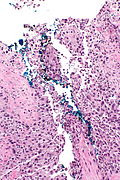
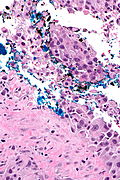
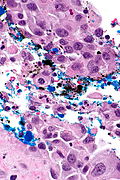
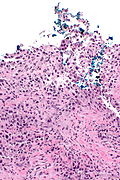
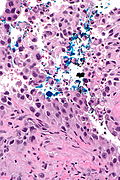
High grade urothelial carcinoma in a 43 year old man. A. At low power, necrosis is seen, luminal, with viable invasive tumor elsewhere. B. Tumor partly fills right ureteral orifice. C. Tumor cells sometimes form Indian files (black arrows), appear to have nuclei that mold (red arrows), and have granular chromatin (cyan arrows), raising possibility of neuroendocrine carcinoma. D. Tumor invades lymphatic spaces. E. Urothelial carcinoma in situ is present. F,G,H. Tumor cells are diffusely positive for CK7, focally positive for CDX2, and diffusely positive for P40, with no positivity for chromogranin or synaptophysin. | |||
=[[IHC]]= | =[[IHC]]= | ||
| Line 120: | Line 131: | ||
:''See [[urothelial carcinoma in situ]]''. | :''See [[urothelial carcinoma in situ]]''. | ||
===UCC versus other=== | ===UCC versus other cancers=== | ||
UCC vs. [[Prostate cancer|prostate]]: | UCC vs. [[Prostate cancer|prostate]]: | ||
*UCC: GATA3 +ve, PSA -ve, [[p63]] +ve, [[CK20]] +ve. | *UCC: GATA3 +ve, PSA -ve, [[p63]] +ve, [[CK20]] +ve. | ||
*Prostate: [[PSA]] +ve, [[GATA3]] -ve, [[PSAP]] +ve, CK7 -ve, CK20 -ve, p63 -ve. | *Prostate: [[PSA]] +ve, [[GATA3]] -ve, [[PSAP]] +ve, CK7 -ve, CK20 -ve, p63 -ve. | ||
UCC vs. [[renal cell carcinoma| | UCC vs. [[renal cell carcinoma|renal cell carcinoma]]: | ||
*UCC: p63 +ve.<ref>{{Cite journal | last1 = Langner | first1 = C. | last2 = Ratschek | first2 = M. | last3 = Tsybrovskyy | first3 = O. | last4 = Schips | first4 = L. | last5 = Zigeuner | first5 = R. | title = P63 immunoreactivity distinguishes upper urinary tract transitional-cell carcinoma and renal-cell carcinoma even in poorly differentiated tumors. | journal = J Histochem Cytochem | volume = 51 | issue = 8 | pages = 1097-9 | month = Aug | year = 2003 | doi = | PMID = 12871991 }} | *UCC: p63 +ve.<ref>{{Cite journal | last1 = Langner | first1 = C. | last2 = Ratschek | first2 = M. | last3 = Tsybrovskyy | first3 = O. | last4 = Schips | first4 = L. | last5 = Zigeuner | first5 = R. | title = P63 immunoreactivity distinguishes upper urinary tract transitional-cell carcinoma and renal-cell carcinoma even in poorly differentiated tumors. | journal = J Histochem Cytochem | volume = 51 | issue = 8 | pages = 1097-9 | month = Aug | year = 2003 | doi = | PMID = 12871991 }} | ||
</ref> | </ref> | ||
Metastatic UCC versus primary lung | Metastatic UCC versus primary lung squamous cell carcinoma: | ||
:See ''[[Squamous_cell_carcinoma_of_the_lung#Lung_SCC_versus_metastatic_bladder_urothelial_carcinoma]]''. | |||
Note: | Note: | ||
| Line 138: | Line 149: | ||
===IHC for staging=== | ===IHC for staging=== | ||
*''Smoothelin'' immunostain for [[bladder muscularis propria invasion|muscularis propria invasion]] versus muscularis mucosae invasion - see ''[[Muscularis_propria_invasion_in_the_urinary_bladder#IHC]]''. | |||
=Molecular= | =Molecular= | ||
*Molecular testing usually ''not'' used for diagnosis. | |||
**Molecular subtyping can be approximated with immunostaining - see ''[[Classification of urothelial carcinoma by immunohistochemistry]]''. | |||
Changes: | Changes: | ||
Latest revision as of 12:54, 13 June 2024
Urothelial carcinoma, also urothelial cell carcinoma, is a malignancy that arises from the urothelium. Urothelial carcinoma is abbreviated UC and urothelial cell carcinoma is abbreviated UCC.
This article deals with flat invasive urothelial carcinoma. The direct precursor is dealt with in urothelial carcinoma in situ.
Papillary urothelial carcinomas are dealt with in low-grade papillary urothelial carcinoma and high-grade papillary urothelial carcinoma.
See urine cytology for the cytopathology.
General
- These lesions lack papillae and are typical flat.
- Clinically, it may not be possible to differentiate renal pelvis urothelial carcinoma and renal cell carcinoma.
- May be a part of Lynch syndrome.
Prognosis:
- Women often have worse outcomes as they present with more advanced tumours.[1]
- Positive soft tissue margin.[2]
- Definition (radical cystectomy): tumour touching ink.
Risk factors:
- Smoking.
- Toxins.
- Drugs, e.g. cyclophosphamide.
- Others.
Microscopic
Features:
- Nuclear pleomorphism - key feature.
- Compare nuclei to one another.
- Increased N/C ratio.
- Lack of maturation to surface (important).
- Cells become dyscohesive.
Invasion vs. in situ: Useful features - present in invasion:[3]
- Thin-walled vessels.
- Stromal reaction (hypercellularity).
- Retraction artefact around the tumour cell nests.
Note:
- The presence/absence of muscle should be commented on in biopsy specimens.
- Adipose tissue may be seen in the lamina propria; tumour adjacent to adipose tissue on a biopsy does not imply invasion deep to the muscularis propria.[4]
DDx:
- Pseudocarcinomatous urothelial hyperplasia.
- Urothelial carcinoma in situ.
- High-grade papillary urothelial carcinoma.
- Low-grade papillary urothelial carcinoma.
- Prostate carcinoma - may have pseudopapillae[5] - see urothelial carcinoma-like prostatic carcinoma.
- Epithelioid angiosarcoma - have intracytoplasmic lumens and interspersed red blood cells, usually have a history radiation treatment.[6]
Staging
- T1 - lamina propria.
- Several subdivisions of T1 exist:
- T1a - superficial or in muscularis mucosae.
- T1b - beyond muscularis mucosae - into submucosa.
- Several subdivisions of T1 exist:
- T2 - muscularis propria.
Note:
- Approximately 25% of muscle invasive urothelial carcinoma on biopsy is a lower stage in the cystectomy specimen.[7]
- In approximately 15% of cases it is pT0 (no primary tumour identified).
Muscularis propria invasion
Images
www:



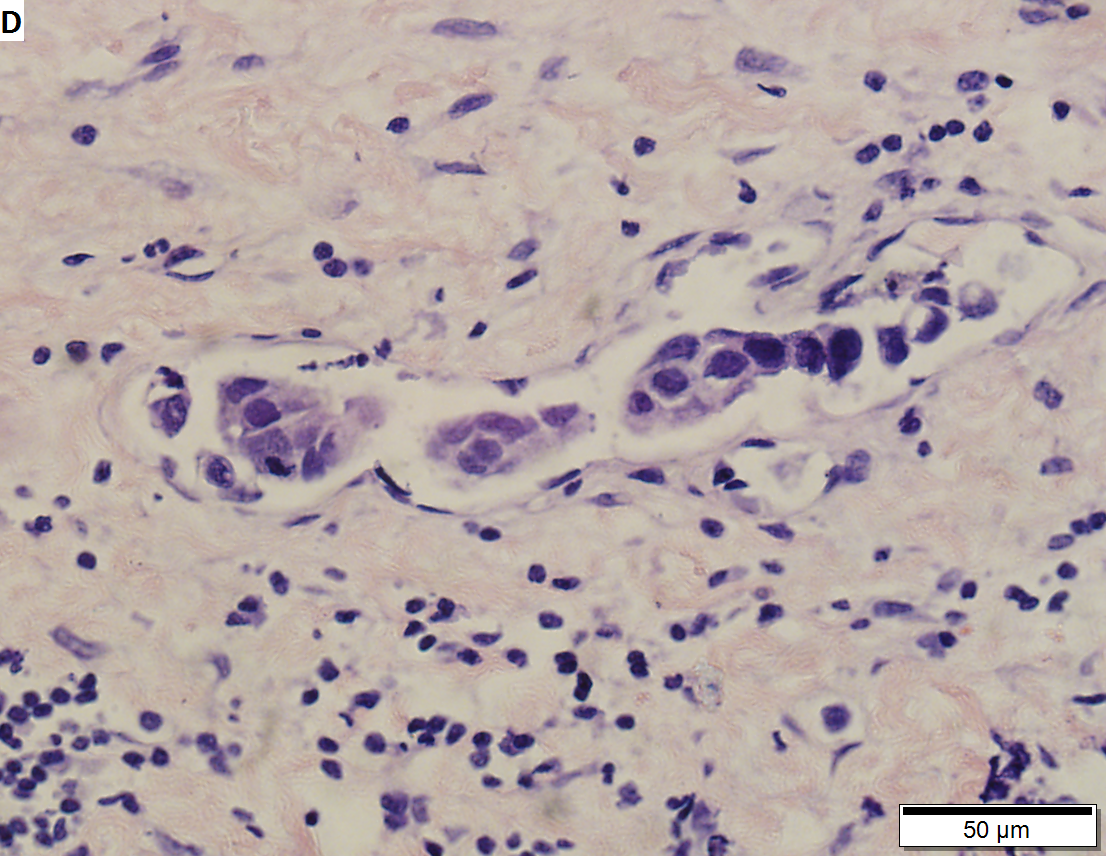

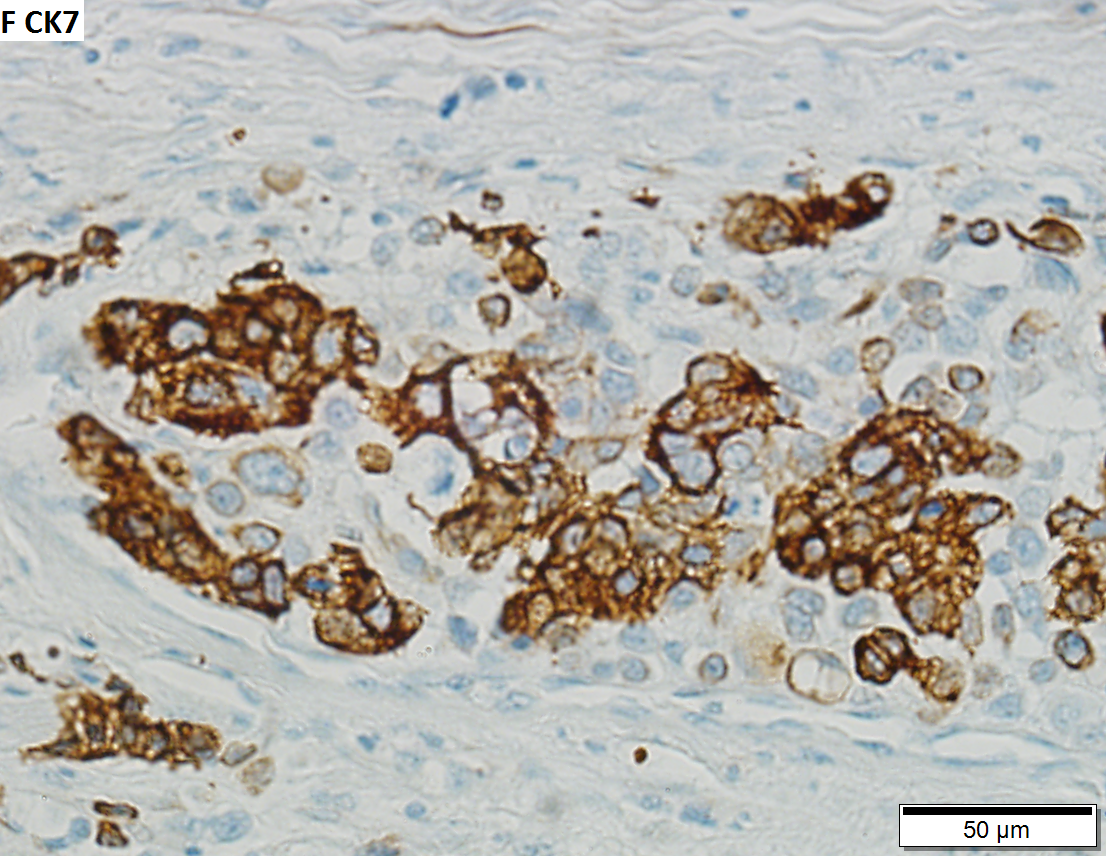

High grade urothelial carcinoma in a 43 year old man. A. At low power, necrosis is seen, luminal, with viable invasive tumor elsewhere. B. Tumor partly fills right ureteral orifice. C. Tumor cells sometimes form Indian files (black arrows), appear to have nuclei that mold (red arrows), and have granular chromatin (cyan arrows), raising possibility of neuroendocrine carcinoma. D. Tumor invades lymphatic spaces. E. Urothelial carcinoma in situ is present. F,G,H. Tumor cells are diffusely positive for CK7, focally positive for CDX2, and diffusely positive for P40, with no positivity for chromogranin or synaptophysin.
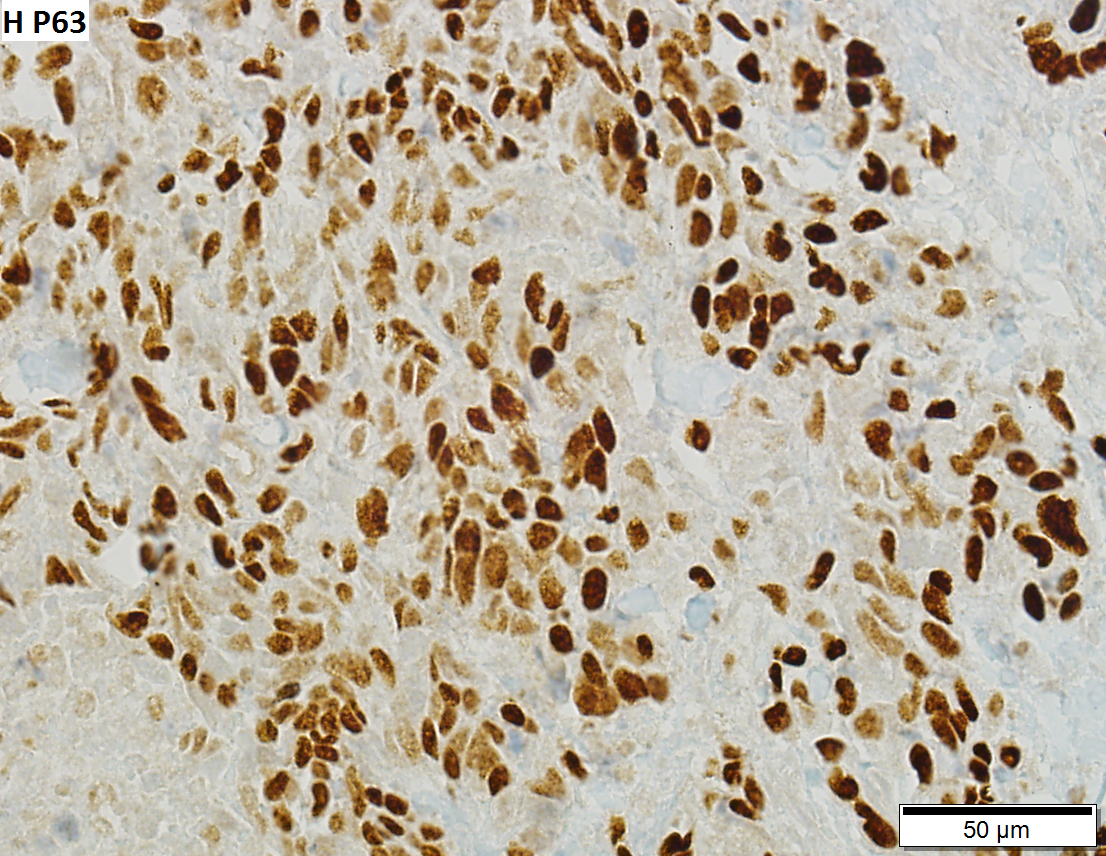
IHC
Recommended by ISUP consensus panel:[9]
- GATA3 +ve, CK20 +ve, p63 +ve, CK5/6, HMWCK (e.g. CK34betaE12) +ve.
Others:
- CK7 +ve.
- PSA -ve.
Notes:
- CK20 negative in over 50% of cases with metastases.[10]
Reactive changes versus UCIS
UCC versus other cancers
UCC vs. prostate:
- UCC: GATA3 +ve, PSA -ve, p63 +ve, CK20 +ve.
- Prostate: PSA +ve, GATA3 -ve, PSAP +ve, CK7 -ve, CK20 -ve, p63 -ve.
UCC vs. renal cell carcinoma:
- UCC: p63 +ve.[11]
Metastatic UCC versus primary lung squamous cell carcinoma:
Note:
- In a large series, PSA positivity is reported in 1.4% bladder UCC.[12]
- In half the cases the staining is weak and in the other half it is strong.[12]
IHC for staging
- Smoothelin immunostain for muscularis propria invasion versus muscularis mucosae invasion - see Muscularis_propria_invasion_in_the_urinary_bladder#IHC.
Molecular
- Molecular testing usually not used for diagnosis.
- Molecular subtyping can be approximated with immunostaining - see Classification of urothelial carcinoma by immunohistochemistry.
Changes:
- 9p deletion -- site of CDKN2A[13] (AKA p16).
- 17p deletion -- site of PT53 (AKA p53).
Sign out
High grade UC
URINARY BLADDER LESION ("TUMOUR"), TRANSURETHRAL RESECTION URINARY BLADDER TUMOUR (TURBT):
- INVASIVE HIGH-GRADE PAPILLARY UROTHELIAL CARCINOMA WITH SQUAMOUS DIFFERENTIATION AT LEAST
INTO MUSCULARIS PROPRIA.
- LYMPHOVASCULAR INVASION PRESENT.
UCC with some suspicion for muscularis propria invasion
URINARY BLADDER LESION ("TUMOUR"), DEEP, RE-RESECTION (TURBT):
- INVASIVE HIGH-GRADE UROTHELIAL CARCINOMA WITH SQUAMOUS DIFFERENTIATION AT
LEAST INTO THE LAMINA PROPRIA, SEE COMMENT.
- NO DEFINITE LYMPHOVASCULAR INVASION.
COMMENT:
Tumour is seen adjacent to smooth muscle fibres of intermediate thickness. This is
interpreted as thick muscularis mucosae. The tissue orientation is suboptimal.
Definite muscularis propria is not apparent. Levels were cut.
Tumour is abundant in the lamina propria.
Alternate comment
The sections shows thickened muscle bundles with frayed edges between the tumour cells. The muscle is thought to represent hypertrophic muscularis mucosae. The large extent of lamina propria invasion raises the possibility of a higher stage lesion that may not have been sampled.
Subtypes of urothelial carcinoma
There are numerous subtypes:[14]
- Squamous differentiation.
- Clear cell.
- Plasmacytoid.
- Micropapillary.
- Small nests (< ~10 cells/nest).
- Sarcomatoid.
- Many others...
Benign patterns - mnemonic Much GIN:
- Microcystic.
- Small tubular/glandular.
- Inverted.
- Nested.
Plasmacytoid urothelial cell carcinoma
Microcystic urothelial carcinoma
Micropapillary urothelial carcinoma
Lymphoepithelioma-like carcinoma
Nested urothelial cell carcinoma
- AKA nested variant of urothelial cell carcinoma.
See also
References
- ↑ Mitra, AP.; Skinner, EC.; Schuckman, AK.; Quinn, DI.; Dorff, TB.; Daneshmand, S. (Jan 2014). "Effect of gender on outcomes following radical cystectomy for urothelial carcinoma of the bladder: a critical analysis of 1,994 patients.". Urol Oncol 32 (1): 52.e1-9. doi:10.1016/j.urolonc.2013.08.007. PMID 24239476.
- ↑ Dotan, ZA.; Kavanagh, K.; Yossepowitch, O.; Kaag, M.; Olgac, S.; Donat, M.; Herr, HW. (Dec 2007). "Positive surgical margins in soft tissue following radical cystectomy for bladder cancer and cancer specific survival.". J Urol 178 (6): 2308-12; discussion 2313. doi:10.1016/j.juro.2007.08.023. PMID 17936804.
- ↑ Sternberg, SE. Histology for Pathologists. P.2047.
- ↑ Bochner, BH.; Nichols, PW.; Skinner, DG. (Mar 1995). "Overstaging of transitional cell carcinoma: clinical significance of lamina propria fat within the urinary bladder.". Urology 45 (3): 528-31. doi:10.1016/S0090-4295(99)80030-2. PMID 7879346.
- ↑ Gordetsky J, Epstein JI (July 2014). "Pseudopapillary features in prostatic adenocarcinoma mimicking urothelial carcinoma: a diagnostic pitfall". Am. J. Surg. Pathol. 38 (7): 941–5. doi:10.1097/PAS.0000000000000178. PMID 24503758.
- ↑ Matoso A, Epstein JI (October 2015). "Epithelioid Angiosarcoma of the Bladder: A Series of 9 Cases". Am J Surg Pathol 39 (10): 1377–82. doi:10.1097/PAS.0000000000000444. PMID 25929352.
- ↑ D'Souza, AM.; Pohar, KS.; Arif, T.; Geyer, S.; Zynger, DL. (Oct 2012). "Retrospective analysis of survival in muscle-invasive bladder cancer: impact of pT classification, node status, lymphovascular invasion, and neoadjuvant chemotherapy.". Virchows Arch 461 (4): 467-74. doi:10.1007/s00428-012-1249-4. PMID 22915241.
- ↑ Terada, T. (Oct 2011). "Nested variant of urothelial carcinoma of the urinary bladder.". Rare Tumors 3 (4): e42. doi:10.4081/rt.2011.e42. PMC 3282447. PMID 22355497. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3282447/.
- ↑ Amin MB, Epstein JI, Ulbright TM, et al. (August 2014). "Best practices recommendations in the application of immunohistochemistry in urologic pathology: report from the international society of urological pathology consensus conference". Am. J. Surg. Pathol. 38 (8): 1017–22. doi:10.1097/PAS.0000000000000254. PMID 25025364.
- ↑ Jiang, J.; Ulbright, TM.; Younger, C.; Sanchez, K.; Bostwick, DG.; Koch, MO.; Eble, JN.; Cheng, L. (Jul 2001). "Cytokeratin 7 and cytokeratin 20 in primary urinary bladder carcinoma and matched lymph node metastasis.". Arch Pathol Lab Med 125 (7): 921-3. doi:10.1043/0003-9985(2001)1250921:CACIPU2.0.CO;2. PMID 11419977.
- ↑ Langner, C.; Ratschek, M.; Tsybrovskyy, O.; Schips, L.; Zigeuner, R. (Aug 2003). "P63 immunoreactivity distinguishes upper urinary tract transitional-cell carcinoma and renal-cell carcinoma even in poorly differentiated tumors.". J Histochem Cytochem 51 (8): 1097-9. PMID 12871991.
- ↑ 12.0 12.1 Chen, JC.; Ho, CL.; Tsai, HW.; Tzai, TS.; Liu, HS.; Chow, NH.; Yang, WH.; Cheng, HL.. "Immunohistochemical detection of prostate-specific antigen expression in primary urothelial carcinoma of the urinary bladder.". Anticancer Res 28 (6B): 4149-54. PMID 19192675.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 600160
- ↑ URL: http://www.nature.com/modpathol/journal/v22/n2s/full/modpathol200926a.html. Accessed on: 19 August 2011.