Difference between revisions of "Estrogen receptor"
Jump to navigation
Jump to search
| (10 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{ Infobox immunostain | |||
| Name = {{PAGENAME}} | |||
| Image = Stomach Metastatic Breast Carcinoma, IHC for Estrogen Receptor (5610737866).jpg | |||
| Width = | |||
| Caption = ER+ve in metastatic breast carcinoma. | |||
| Abbrev = ER | |||
| Synonyms = | |||
| Similar = | |||
| Clones = | |||
| Use = | |||
| Subspecial = | |||
| Pattern = nuclear staining | |||
| Positive = Breast and endometrial carcinoma | |||
| Negative = | |||
| Other = | |||
}} | |||
'''Estrogen receptor''', abbreviated '''ER''', is a common [[immunostain]]. | '''Estrogen receptor''', abbreviated '''ER''', is a common [[immunostain]]. | ||
==General== | ==General== | ||
In the context of breast pathology it is a [[class II IHC test]], as it is used for treatment decisions by itself.<ref name=pmid20154273>{{Cite journal | last1 = Torlakovic | first1 = EE. | last2 = Riddell | first2 = R. | last3 = Banerjee | first3 = D. | last4 = El-Zimaity | first4 = H. | last5 = Pilavdzic | first5 = D. | last6 = Dawe | first6 = P. | last7 = Magliocco | first7 = A. | last8 = Barnes | first8 = P. | last9 = Berendt | first9 = R. | title = Canadian Association of Pathologists-Association canadienne des pathologistes National Standards Committee/Immunohistochemistry: best practice recommendations for standardization of immunohistochemistry tests. | journal = Am J Clin Pathol | volume = 133 | issue = 3 | pages = 354-65 | month = Mar | year = 2010 | doi = 10.1309/AJCPDYZ1XMF4HJWK | PMID = 20154273 }}</ref> | *Nuclear stain - like most other "p" stains. | ||
*In the context of breast pathology it is a [[Quality#Classification_of_IHC_tests|class II IHC test]], as it is used for treatment decisions by itself.<ref name=pmid20154273>{{Cite journal | last1 = Torlakovic | first1 = EE. | last2 = Riddell | first2 = R. | last3 = Banerjee | first3 = D. | last4 = El-Zimaity | first4 = H. | last5 = Pilavdzic | first5 = D. | last6 = Dawe | first6 = P. | last7 = Magliocco | first7 = A. | last8 = Barnes | first8 = P. | last9 = Berendt | first9 = R. | title = Canadian Association of Pathologists-Association canadienne des pathologistes National Standards Committee/Immunohistochemistry: best practice recommendations for standardization of immunohistochemistry tests. | journal = Am J Clin Pathol | volume = 133 | issue = 3 | pages = 354-65 | month = Mar | year = 2010 | doi = 10.1309/AJCPDYZ1XMF4HJWK | PMID = 20154273 }}</ref> | |||
==Positive== | ==Positive== | ||
*Normal breast - usually patchy staining. | |||
*[[Florid epithelial hyperplasia of the usual type]] - usually patchy staining<ref name=Ref_BP276>{{Ref BP|276}}</ref> - see ''[[Invasive_breast_cancer#Immunostains_for_typing_and_diagnosis|immunostains for typing and diagnosis of breast lesions]]''. | |||
*[[Breast carcinoma]] - most. | *[[Breast carcinoma]] - most. | ||
**[[Ductal carcinoma in situ]] - most. | **[[Ductal carcinoma in situ]] - most, usually diffuse<ref name=Ref_BP276>{{Ref BP|276}}</ref> - see ''[[Invasive_breast_cancer#Immunostains_for_typing_and_diagnosis|immunostains for typing and diagnosis of breast lesions]]''. | ||
**[[Invasive lobular carcinoma]] - all. | **[[Invasive lobular carcinoma]] - all. | ||
**[[Invasive ductal carcinoma of the breast]] - most. | **[[Invasive ductal carcinoma of the breast]] - most. | ||
*[[Endometrial carcinoma]]s. | *[[Endometrial carcinoma]]s. | ||
* PRL-producing [[pituitary adenoma]]s (Prolactioma)<ref>{{Cite journal | last1 = Delgrange | first1 = E. | last2 = Vasiljevic | first2 = A. | last3 = Wierinckx | first3 = A. | last4 = François | first4 = P. | last5 = Jouanneau | first5 = E. | last6 = Raverot | first6 = G. | last7 = Trouillas | first7 = J. | title = Expression of estrogen receptor alpha is associated with prolactin pituitary tumor prognosis and supports the sex-related difference in tumor growth. | journal = Eur J Endocrinol | volume = 172 | issue = 6 | pages = 791-801 | month = Jun | year = 2015 | doi = 10.1530/EJE-14-0990 | PMID = 25792376 }}</ref> | |||
===Occasionally positive=== | |||
*[[Lung adenocarcinoma]] - dependent on the ER subunit target - ERα ~1%, ERβ ~79%.<ref name=pmid27069542>{{Cite journal | last1 = Tanaka | first1 = K. | last2 = Shimizu | first2 = K. | last3 = Kakegawa | first3 = S. | last4 = Ohtaki | first4 = Y. | last5 = Nagashima | first5 = T. | last6 = Kaira | first6 = K. | last7 = Horiguchi | first7 = J. | last8 = Oyama | first8 = T. | last9 = Takeyoshi | first9 = I. | title = Prognostic significance of aromatase and estrogen receptor beta expression in EGFR wild-type lung adenocarcinoma. | journal = Am J Transl Res | volume = 8 | issue = 1 | pages = 81-97 | month = | year = 2016 | doi = | PMID = 27069542 }}</ref> | |||
==Negative== | ==Negative== | ||
*[[Endocervical adenocarcinoma]]. | *[[Endocervical adenocarcinoma]].<ref name=pmid20335127>{{Cite journal | last1 = Hu | first1 = WW. | last2 = Tao | first2 = JH. | last3 = Li | first3 = GM. | last4 = Xu | first4 = X. | last5 = Yang | first5 = XM. | title = [Value of ER, VIM, CEA and p16 detection in the diagnosis and differential diagnosis of primary endocervical and endometrial adenocarcinomas]. | journal = Nan Fang Yi Ke Da Xue Xue Bao | volume = 30 | issue = 3 | pages = 526-8, 531 | month = Mar | year = 2010 | doi = | PMID = 20335127 }}</ref> | ||
==See also== | ==See also== | ||
*[[Breast pathology]]. | *[[Breast pathology]]. | ||
*[[Tamoxifen]]. | |||
==References== | ==References== | ||
Latest revision as of 18:27, 30 August 2023
| Estrogen receptor | |
|---|---|
| Immunostain in short | |
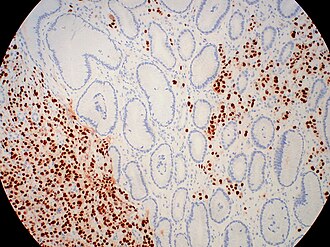 ER+ve in metastatic breast carcinoma. | |
| Abbreviation | ER |
| Normal staining pattern | nuclear staining |
| Positive | Breast and endometrial carcinoma |
Estrogen receptor, abbreviated ER, is a common immunostain.
General
- Nuclear stain - like most other "p" stains.
- In the context of breast pathology it is a class II IHC test, as it is used for treatment decisions by itself.[1]
Positive
- Normal breast - usually patchy staining.
- Florid epithelial hyperplasia of the usual type - usually patchy staining[2] - see immunostains for typing and diagnosis of breast lesions.
- Breast carcinoma - most.
- Ductal carcinoma in situ - most, usually diffuse[2] - see immunostains for typing and diagnosis of breast lesions.
- Invasive lobular carcinoma - all.
- Invasive ductal carcinoma of the breast - most.
- Endometrial carcinomas.
- PRL-producing pituitary adenomas (Prolactioma)[3]
Occasionally positive
- Lung adenocarcinoma - dependent on the ER subunit target - ERα ~1%, ERβ ~79%.[4]
Negative
See also
References
- ↑ Torlakovic, EE.; Riddell, R.; Banerjee, D.; El-Zimaity, H.; Pilavdzic, D.; Dawe, P.; Magliocco, A.; Barnes, P. et al. (Mar 2010). "Canadian Association of Pathologists-Association canadienne des pathologistes National Standards Committee/Immunohistochemistry: best practice recommendations for standardization of immunohistochemistry tests.". Am J Clin Pathol 133 (3): 354-65. doi:10.1309/AJCPDYZ1XMF4HJWK. PMID 20154273.
- ↑ 2.0 2.1 O'Malley, Frances P.; Pinder, Sarah E. (2006). Breast Pathology: A Volume in Foundations in Diagnostic Pathology series (1st ed.). Churchill Livingstone. pp. 276. ISBN 978-0443066801.
- ↑ Delgrange, E.; Vasiljevic, A.; Wierinckx, A.; François, P.; Jouanneau, E.; Raverot, G.; Trouillas, J. (Jun 2015). "Expression of estrogen receptor alpha is associated with prolactin pituitary tumor prognosis and supports the sex-related difference in tumor growth.". Eur J Endocrinol 172 (6): 791-801. doi:10.1530/EJE-14-0990. PMID 25792376.
- ↑ Tanaka, K.; Shimizu, K.; Kakegawa, S.; Ohtaki, Y.; Nagashima, T.; Kaira, K.; Horiguchi, J.; Oyama, T. et al. (2016). "Prognostic significance of aromatase and estrogen receptor beta expression in EGFR wild-type lung adenocarcinoma.". Am J Transl Res 8 (1): 81-97. PMID 27069542.
- ↑ Hu, WW.; Tao, JH.; Li, GM.; Xu, X.; Yang, XM. (Mar 2010). "[Value of ER, VIM, CEA and p16 detection in the diagnosis and differential diagnosis of primary endocervical and endometrial adenocarcinomas].". Nan Fang Yi Ke Da Xue Xue Bao 30 (3): 526-8, 531. PMID 20335127.