Difference between revisions of "Neurodegenerative diseases"
Jensflorian (talk | contribs) (→Alzheimer disease: update) |
m (vauthors -> authors) |
||
| (40 intermediate revisions by one other user not shown) | |||
| Line 13: | Line 13: | ||
{{familytree | | | | | | | A01 | | | | | | | | A01=Neurodegenerative<br>disorders}} | {{familytree | | | | | | | A01 | | | | | | | | A01=Neurodegenerative<br>disorders}} | ||
{{familytree | |,|-|-|-|v|-|^|-|v|-|-|-|v|-|-|-|.| | |}} | {{familytree | |,|-|-|-|v|-|^|-|v|-|-|-|v|-|-|-|.| | |}} | ||
{{familytree | B01 | | B02 | | B03 | | B04 | | B05 || B01=Amyloidoses|B02=Tauopathies|B03=α-synucleinopathies|B04=TDP-43|B05=FUS}} | {{familytree | B01 | | B02 | | B03 | | B04 | | B05 || B01=Amyloidoses|B02=Tauopathies|B03=α-synucleinopathies|B04=TDP-43|B05=FUS/EWS/TAF15}} | ||
{{familytree/end}} | {{familytree/end}} | ||
| Line 34: | Line 34: | ||
TDP-43 proteinopathies: | TDP-43 proteinopathies: | ||
*[[Amyotrophic lateral sclerosis]]. | *[[Amyotrophic lateral sclerosis]]. | ||
*Frontotemporal lobar degeneration. | *Frontotemporal lobar degeneration with TDP-43 (FTLD-TDP). | ||
FET proteinopathies: | |||
*Basophilic inclusion body disease (BIBD). | *Basophilic inclusion body disease (BIBD). | ||
*Neuronal intermediate filament inclusion disease (NIFID). | *Neuronal intermediate filament inclusion disease (NIFID). | ||
* | *Atypical frontotemporal lobar degeneration with ubiquitin-positive inclusions (atypical FTLD-U). | ||
Prionopathies: | Prionopathies: | ||
*Creutzfeldt-Jakob disease (PrP). | *Creutzfeldt-Jakob disease (PrP). | ||
'''Note:''' Some people consider α-synuclein as a prion-like protein.<ref>{{Cite journal | last1 = Watts | first1 = JC. | title = Calling α-synuclein a prion is scientifically justifiable. | journal = Acta Neuropathol | volume = 138 | issue = 4 | pages = 505-508 | month = Oct | year = 2019 | doi = 10.1007/s00401-019-02058-0 | PMID = 31407029 }}</ref> | |||
====Table==== | ====Table==== | ||
| Line 48: | Line 50: | ||
{| class="wikitable sortable" style="margin-left:auto;margin-right:auto" | {| class="wikitable sortable" style="margin-left:auto;margin-right:auto" | ||
! Disease | ! Disease | ||
! | ! Deposited protein | ||
! Distribution | ! Distribution | ||
! Clinical | ! Clinical | ||
| Line 90: | Line 92: | ||
|- | |- | ||
| [[Amyotrophic lateral sclerosis|Amyotrophic lateral <br>sclerosis (ALS)]] | | [[Amyotrophic lateral sclerosis|Amyotrophic lateral <br>sclerosis (ALS)]] | ||
| TDP-43 | | [[TDP-43]] | ||
| motor neurons | | motor neurons | ||
| spasticity, weakness | | spasticity, weakness | ||
| Line 97: | Line 99: | ||
|- | |- | ||
| Frontotemporal lobar <br>degeneration with TDP-43 (FTLD-TDP) | | Frontotemporal lobar <br>degeneration with TDP-43 (FTLD-TDP) | ||
| TDP-43 | | [[TDP-43]] | ||
| cortex, basal ganglia | | cortex, basal ganglia | ||
| dementia, focal cortical syndromes | | dementia, focal cortical syndromes | ||
| histology depends on (type 1-4), ubiquitin and TDP-43+ve, tau and FUS-ve | | histology depends on (type 1-4), ubiquitin and [[TDP-43]]+ve, tau and FUS-ve | ||
| [https://commons.wikimedia.org/wiki/File:FTLD_TSP43_hippocampus.jpg] | | [https://commons.wikimedia.org/wiki/File:FTLD_TSP43_hippocampus.jpg] | ||
|- | |- | ||
| Frontotemporal lobar <br>degeneration with | | Frontotemporal lobar <br>degeneration with FET (FTLD-FET) | ||
| FUS | | FUS/EWS/TAF15 | ||
| cortex, medulla, hippocampus, and motor cells of the spinal cord | | cortex, medulla, hippocampus, and motor cells of the spinal cord | ||
| dementia, cases classified as | | dementia, cases classified as aFTLD-U, NIFID and BIBD | ||
| FUS+ve, TAF15+ve, EWS+ve cytoplasmic & intranuclear inclusions, neuritic threads | | FUS+ve, TAF15+ve, EWS+ve cytoplasmic & intranuclear inclusions, neuritic threads | ||
| [http://brain.oxfordjournals.org/content/brain/134/9/2595/F1.medium.gif] | | [http://brain.oxfordjournals.org/content/brain/134/9/2595/F1.medium.gif] | ||
| Line 150: | Line 152: | ||
===Alpha-synuclein=== | ===Alpha-synuclein=== | ||
Look for: | Look for: | ||
*Lewy bodies (seen in Parkinson's | *Lewy bodies (seen in Parkinson's Disease (PD), Dementia with Lewy bodies (DLB)) = round cytoplasmic eosinophilic body +/- pale halo. | ||
*Glial cytoplasmatic inclusions (Papp-Lantos bodies) seen in MSA. | *Lewy neurites(seen in [[PD]] and [[DLB]]) = abnormal neurites with filaments similar to those found in Lewy bodies. | ||
*Glial cytoplasmatic inclusions (Papp-Lantos bodies) seen in mutisystem atrophy (MSA). | |||
*Beta amyloid in vessels seen in cerebral amyloid angiopathy (CAA). | |||
===Tau=== | ===Tau=== | ||
*AT8 = stains phosphorylated tau.<ref name=pmid19946779>{{cite journal |author=Seelaar H, Klijnsma KY, de Koning I, ''et al.'' |title=Frequency of ubiquitin and FUS-positive, TDP-43-negative frontotemporal lobar degeneration |journal=J. Neurol. |volume=257 |issue=5 |pages=747–53 |year=2010 |month=May |pmid=19946779 |pmc=2864899 |doi=10.1007/s00415-009-5404-z |url=http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2864899/}}</ref> | *AT8 = stains phosphorylated tau.<ref name=pmid19946779>{{cite journal |author=Seelaar H, Klijnsma KY, de Koning I, ''et al.'' |title=Frequency of ubiquitin and FUS-positive, [[TDP-43]]-negative frontotemporal lobar degeneration |journal=J. Neurol. |volume=257 |issue=5 |pages=747–53 |year=2010 |month=May |pmid=19946779 |pmc=2864899 |doi=10.1007/s00415-009-5404-z |url=http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2864899/}}</ref> | ||
**''AT'' = anti-tau. | **''AT'' = anti-tau. | ||
**Stains tau 4R and tau 3R.<ref>{{cite journal |author=Kumaran R, Kingsbury A, Coulter I, ''et al.'' |title=DJ-1 (PARK7) is associated with 3R and 4R tau neuronal and glial inclusions in neurodegenerative disorders |journal=Neurobiol. Dis. |volume=28 |issue=1 |pages=122–32 |year=2007 |month=October |pmid=17719794 |doi=10.1016/j.nbd.2007.07.012 |url=}}</ref> | **Stains tau 4R and tau 3R.<ref>{{cite journal |author=Kumaran R, Kingsbury A, Coulter I, ''et al.'' |title=DJ-1 (PARK7) is associated with 3R and 4R tau neuronal and glial inclusions in neurodegenerative disorders |journal=Neurobiol. Dis. |volume=28 |issue=1 |pages=122–32 |year=2007 |month=October |pmid=17719794 |doi=10.1016/j.nbd.2007.07.012 |url=}}</ref> | ||
| Line 170: | Line 174: | ||
===Ubiquitin=== | ===Ubiquitin=== | ||
*Marks proteins for recycling. | *Marks proteins for recycling. | ||
*Stains Barr bodies in hippocampal granule cells<ref> {{Cite journal | last1 = Gelpi | first1 = E. | title = Clinical Neuropathology teaching case 3-2015: female or male brain? Anti-ubiquitin visualizes Barr bodies in hippocampal granule cells which allows the determination of gender in human brains. | journal = Clin Neuropathol | volume = 34 | issue = 3 | pages = 115-6 | month = | year = | doi = | PMID = 25909954 }}</ref> | |||
=== | |||
===p62=== | |||
*p62; poli-ubiquitin-binding protein p62.<ref name=pmid19946779/> | *p62; poli-ubiquitin-binding protein p62.<ref name=pmid19946779/> | ||
===Microscopic=== | |||
Look for: | Look for: | ||
*Lewy bodies. ( | * Lewy bodies and extracellular pigment in neuromelanin-containing nuclei (SN, LC, DVN) -> PD. | ||
* Spongiform vacuolation in the neuropil (seen in Prion disease and FTLD-TDP). | |||
* Neurofibrillar tangles (pyramidal layer of dentate gyrus). | |||
* Granulovacuolar degeneration (granules within cytoplasmic vacuoles, mainly in the hippocampal pyramidal neurons, seen in AD). | |||
* Cores of amyloid plaqyes. | |||
* Cotton wool plaques (seen in familiar AD). | |||
* Pick cells (balloned neurons in frontal cortex). | |||
* Pick bodies (granular layer of dentate gyrus). | |||
* Extensive astrogliosis (striatonigral degeneration, hepatic encephalopathy). | |||
* Corpora amylacea in the cornu ammonis may be increased in neurodegenerative diseases. <ref>{{Cite journal | last1 = Kovacs | first1 = GG. | last2 = Risser | first2 = D. | title = Clinical Neuropathology image 6-2014: Corpora amylacea replacing cornu ammonis (CACA). | journal = Clin Neuropathol | volume = 33 | issue = 6 | pages = 378-9 | month = | year = | doi = | PMID = 25343241 }}</ref> | |||
<gallery> | |||
File:213-09-11-Congo Red Lewy body.tif|Lewy body | |||
File:Amyloid plaques alzheimer disease HE stain.jpg|Cotton wool plaques | |||
File:Neurofibrillary tangles in the Hippocampus of an old person with Alzheimer-related pathology, HE 3.JPG|Neurofibrillary tangles | |||
File:SpongiformChangeCJD.jpg | Spongiform vacuolation | |||
</gallery> | |||
=Clinical perspective= | =Clinical perspective= | ||
*Correlations between clinical signs and molecular can be poor. | |||
**Example: The MAPT A152T gene mutation may cause clinical symptoms matching AD, [[Neurodegenerative diseases#Corticobasal degeneration|CBD]], [[Neurodegenerative diseases#Progressive supranuclear palys|PSP]] and [[Neurodegenerative diseases#Lewy body disease|LBD]].<ref>{{Cite journal | last1 = Coppola | first1 = G. | last2 = Chinnathambi | first2 = S. | last3 = Lee | first3 = JJ. | last4 = Dombroski | first4 = BA. | last5 = Baker | first5 = MC. | last6 = Soto-Ortolaza | first6 = AI. | last7 = Lee | first7 = SE. | last8 = Klein | first8 = E. | last9 = Huang | first9 = AY. | title = Evidence for a role of the rare p.A152T variant in MAPT in increasing the risk for FTD-spectrum and Alzheimer's diseases. | journal = Hum Mol Genet | volume = 21 | issue = 15 | pages = 3500-12 | month = Aug | year = 2012 | doi = 10.1093/hmg/dds161 | PMID = 22556362 }}</ref> | |||
===Dementia general (mostly useless) DDx=== | ===Dementia general (mostly useless) DDx=== | ||
*[[Alzheimer's disease|Alzheimer's]] dementia - most common. | *[[Alzheimer's disease|Alzheimer's]] dementia - most common. | ||
| Line 352: | Line 377: | ||
*Misfolded cell-surface protein called PrP<sup>SC</sup>. | *Misfolded cell-surface protein called PrP<sup>SC</sup>. | ||
**This is derived from the protein ''PrP<sup>C</sup>'' encoded by the ''PRNP'' gene. | **This is derived from the protein ''PrP<sup>C</sup>'' encoded by the ''PRNP'' gene. | ||
*Different genetics strains are associated with varying clinical phenotype.<ref>{{Cite journal | last1 = Monari | first1 = L. | last2 = Chen | first2 = SG. | last3 = Brown | first3 = P. | last4 = Parchi | first4 = P. | last5 = Petersen | first5 = RB. | last6 = Mikol | first6 = J. | last7 = Gray | first7 = F. | last8 = Cortelli | first8 = P. | last9 = Montagna | first9 = P. | title = Fatal familial insomnia and familial Creutzfeldt-Jakob disease: different prion proteins determined by a DNA polymorphism. | journal = Proc Natl Acad Sci U S A | volume = 91 | issue = 7 | pages = 2839-42 | month = Mar | year = 1994 | doi = 10.1073/pnas.91.7.2839 | PMID = 7908444 }}</ref> | |||
Includes: | Includes: | ||
| Line 388: | Line 415: | ||
*Spongiform changes may be seen in [[ALS]], [[Alzheimer's disease]] and Lewy body disease (e.g. [[Parkinson disease]]); however, the changes are only in the upper cortex and not diffuse.<ref>{{Ref APBR|419 Q4}}</ref> | *Spongiform changes may be seen in [[ALS]], [[Alzheimer's disease]] and Lewy body disease (e.g. [[Parkinson disease]]); however, the changes are only in the upper cortex and not diffuse.<ref>{{Ref APBR|419 Q4}}</ref> | ||
==== | ===Molecular=== | ||
*The CJD phenotype is associated with a PRNP D178N mutation and valine polymorphism at codon 129 (D178N-129V). | |||
** Note: A Met129 polymorphism will cause Fatal familiar insomnia in the setting of the same PRNP D178N mutation. <ref>{{Cite journal | last1 = Goldfarb | first1 = LG. | last2 = Petersen | first2 = RB. | last3 = Tabaton | first3 = M. | last4 = Brown | first4 = P. | last5 = LeBlanc | first5 = AC. | last6 = Montagna | first6 = P. | last7 = Cortelli | first7 = P. | last8 = Julien | first8 = J. | last9 = Vital | first9 = C. | title = Fatal familial insomnia and familial Creutzfeldt-Jakob disease: disease phenotype determined by a DNA polymorphism. | journal = Science | volume = 258 | issue = 5083 | pages = 806-8 | month = Oct | year = 1992 | doi = 10.1126/science.1439789 | PMID = 1439789 }}</ref> | |||
<gallery> | <gallery> | ||
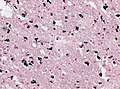
Image:SpongiformChangeCJD.jpg | CJD. (WC/DRdoubleB) | Image:SpongiformChangeCJD.jpg | CJD. (WC/DRdoubleB) | ||
Image:VCJD_Tonsil.jpg | vCJD - prion protein immunostain. (WC/Sbrandner) | File:Variant Creutzfeldt-Jakob disease (vCJD), H&E.jpg|Spongiform changes in CJD. (CDC/ Teresa Hammett) | ||
File:Variant CJD HE.jpg | Florid plaques in vCJD. (WC/Sbrandner) | |||
File:Cdc cjd2.jpg | Florid plaques in vCJD - low mag. (WC/CDC.gov) | |||
Image:VCJD_Tonsil.jpg | vCJD - prion protein immunostain tonsil. (WC/Sbrandner) | |||
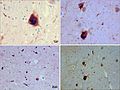
File:CJD PRP cortex.jpg | sCJD - prion protein immunostain cortex. (WC/jensflorian) | |||
File:CJD PRP cerebellum.jpg | sCJD - prion protein immunostain cerebellum. (WC/jensflorian) | |||
</gallery> | </gallery> | ||
*[http://path.upmc.edu/cases/case86.html CJD - several cases (upmc.edu)]. | *[http://path.upmc.edu/cases/case86.html CJD - several cases (upmc.edu)]. | ||
| Line 498: | Line 534: | ||
*Neuronal loss and gliosis (absent in minimal-change MSA). | *Neuronal loss and gliosis (absent in minimal-change MSA). | ||
*Alpha-synuclein-rich glial and neuronal cytoplasmic inclusions in white matter (finding at autopsy).<ref name=pmid18825660>{{Cite journal | last1 = Wenning | first1 = GK. | last2 = Stefanova | first2 = N. | last3 = Jellinger | first3 = KA. | last4 = Poewe | first4 = W. | last5 = Schlossmacher | first5 = MG. | title = Multiple system atrophy: a primary oligodendrogliopathy. | journal = Ann Neurol | volume = 64 | issue = 3 | pages = 239-46 | month = Sep | year = 2008 | doi = 10.1002/ana.21465 | PMID = 18825660 }}</ref> | *Alpha-synuclein-rich glial and neuronal cytoplasmic inclusions in white matter (finding at autopsy).<ref name=pmid18825660>{{Cite journal | last1 = Wenning | first1 = GK. | last2 = Stefanova | first2 = N. | last3 = Jellinger | first3 = KA. | last4 = Poewe | first4 = W. | last5 = Schlossmacher | first5 = MG. | title = Multiple system atrophy: a primary oligodendrogliopathy. | journal = Ann Neurol | volume = 64 | issue = 3 | pages = 239-46 | month = Sep | year = 2008 | doi = 10.1002/ana.21465 | PMID = 18825660 }}</ref> | ||
**Inclusions in oligodendrocytes (triangular, flame-like or sickle-shaped) are definitive diagnostic for MSA.<ref>MUN. 16 November 2010.</ref><ref>{{cite journal | | **Inclusions in oligodendrocytes (triangular, flame-like or sickle-shaped) are definitive diagnostic for MSA.<ref>MUN. 16 November 2010.</ref><ref>{{cite journal |authors=Trojanowski JQ, Revesz T |title=Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy |journal=Neuropathol. Appl. Neurobiol. |volume=33 |issue=6 |pages=615–20 |year=2007 |pmid=17990994 |doi=10.1111/j.1365-2990.2007.00907.x |url=}}</ref> | ||
**Inclusions usu. abundant in basal ganglia, substantia nigra, pontine nuclei, medulla and cerebellum. | **Inclusions usu. abundant in basal ganglia, substantia nigra, pontine nuclei, medulla and cerebellum. | ||
*Pons and Putamen: | *Pons and Putamen: | ||
| Line 517: | Line 553: | ||
=Tauopathies= | =Tauopathies= | ||
More than 20 different degenerative disorders can be classified as tauopathies.<ref>{{Cite journal | last1 = Williams | first1 = DR. | title = Tauopathies: classification and clinical update on neurodegenerative diseases associated with microtubule-associated protein tau. | journal = Intern Med J | volume = 36 | issue = 10 | pages = 652-60 | month = Oct | year = 2006 | doi = 10.1111/j.1445-5994.2006.01153.x | PMID = 16958643 }}</ref> '''FTLD-tau''' is an umbrella term used for tauopathies including PSP, CBD, PiD and GGT. <ref>{{Cite journal | last1 = Forrest | first1 = SL. | last2 = Kril | first2 = JJ. | last3 = Stevens | first3 = CH. | last4 = Kwok | first4 = JB. | last5 = Hallupp | first5 = M. | last6 = Kim | first6 = WS. | last7 = Huang | first7 = Y. | last8 = McGinley | first8 = CV. | last9 = Werka | first9 = H. | title = Retiring the term FTDP-17 as MAPT mutations are genetic forms of sporadic frontotemporal tauopathies. | journal = Brain | volume = 141 | issue = 2 | pages = 521-534 | month = Feb | year = 2018 | doi = 10.1093/brain/awx328 | PMID = 29253099 }}</ref> | |||
==Argyrophilic grain disease== | |||
==Corticobasal degeneration== | |||
*AKA '''CBD'''. | |||
*Symptoms may vary: | |||
**Progressive asymmetrical rigidity and apraxia, progressive aphasia or dementia. | |||
*Neuronal and glial Tau-positive inclusions.<ref>{{Cite journal | last1 = Dickson | first1 = DW. | last2 = Bergeron | first2 = C. | last3 = Chin | first3 = SS. | last4 = Duyckaerts | first4 = C. | last5 = Horoupian | first5 = D. | last6 = Ikeda | first6 = K. | last7 = Jellinger | first7 = K. | last8 = Lantos | first8 = PL. | last9 = Lippa | first9 = CF. | title = Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. | journal = J Neuropathol Exp Neurol | volume = 61 | issue = 11 | pages = 935-46 | month = Nov | year = 2002 | doi = | PMID = 12430710 }}</ref> | |||
**Astrocytic plaques. | |||
**Thread-like lesions and coiled bodies. | |||
**Ballooned neurons +/-. | |||
*Pathology is cortical and striatal and Gallyas-positive. | |||
*Neuronal loss in the substantia nigra. | |||
DD: PSP (widespread neurofibrillary degeneration, with characteristic globose NFT). | |||
==Globular glial tauopathies== | |||
*Commonly abbreviated ''GGT''. | |||
*AKA ''sporadic multiple system tauopathy''. | |||
*Rare disease.<ref>{{Cite journal | last1 = Ahmed | first1 = Z. | last2 = Bigio | first2 = EH. | last3 = Budka | first3 = H. | last4 = Dickson | first4 = DW. | last5 = Ferrer | first5 = I. | last6 = Ghetti | first6 = B. | last7 = Giaccone | first7 = G. | last8 = Hatanpaa | first8 = KJ. | last9 = Holton | first9 = JL. | title = Globular glial tauopathies (GGT): consensus recommendations. | journal = Acta Neuropathol | volume = 126 | issue = 4 | pages = 537-544 | month = Oct | year = 2013 | doi = 10.1007/s00401-013-1171-0 | PMID = 23995422 }}</ref> | |||
*Combination of frontotemporal dementia and motor neuron disease or only part thereof. | |||
*4-repeat tauopathy. | |||
===Microscopic=== | |||
*Globular oligodendroglial and astrocytic Tau inclusions. | |||
*Absence of tufted astrocytes. | |||
*Mostly Gallyas-negative. | |||
==Progressive supranuclear palsy== | ==Progressive supranuclear palsy== | ||
*Commonly abbreviated ''PSP''. | *Commonly abbreviated ''PSP''. | ||
| Line 558: | Line 625: | ||
*[http://www.nature.com/nrneurol/journal/v6/n2/fig_tab/nrneurol.2009.216_F1.html Pick body (nature.com)].<ref name=pmid20139998>{{Cite journal | last1 = Grossman | first1 = M. | title = Primary progressive aphasia: clinicopathological correlations. | journal = Nat Rev Neurol | volume = 6 | issue = 2 | pages = 88-97 | month = Feb | year = 2010 | doi = 10.1038/nrneurol.2009.216 | PMID = 20139998 }}</ref> | *[http://www.nature.com/nrneurol/journal/v6/n2/fig_tab/nrneurol.2009.216_F1.html Pick body (nature.com)].<ref name=pmid20139998>{{Cite journal | last1 = Grossman | first1 = M. | title = Primary progressive aphasia: clinicopathological correlations. | journal = Nat Rev Neurol | volume = 6 | issue = 2 | pages = 88-97 | month = Feb | year = 2010 | doi = 10.1038/nrneurol.2009.216 | PMID = 20139998 }}</ref> | ||
= | =TDP Proteinopathies= | ||
==FTLD-TDP== | |||
*Accounts for about 50% of all FTLD cases. | |||
*Degeneration of frontal and temporal lobes. | |||
*Inclusions not seen in HE or silver stains. | |||
*TDP43-positive | |||
**Neuronal cytoplasmic inclusions. | |||
**Neuronal intranuclear inclusions. | |||
**Dystrophic neurites. | |||
*Ubiquitin+ve. | |||
*p62+ve. | |||
*aSynculein-ve. | |||
*Tau-ve. | |||
*FUS-ve. | |||
*Four FTLD-TDP subtypes | |||
** Type A: compact nuclear/cytoplasmatic inclusions, associated with GRN mutations. | |||
** Type B: diffuse nuclear/cytoplasmatic inclusions most often seen in C9orf72 expansion. | |||
** Type C: dystrophic neurites. | |||
** Type D: Lentiform nuclear inclusions, only in cases with VCP mutations. | |||
*C9orf72 mutated show additional DPR+ve staining of TDP‐43‐ve inclusions. | |||
**These addtional inclusions are ubiquitin+ve and p62+ve | |||
=FTLD-FET= | |||
* Clinical manifestations depend on the distribution of the pathologic alterations in the CNS | * Clinical manifestations depend on the distribution of the pathologic alterations in the CNS | ||
* Currently 3 disorders among the FTLD- | * Currently 3 disorders among the FTLD-FET subgroup. | ||
* In contrast to ALS-FUS, no genetic alterations of FUS have been reported to date for cases within the FTLD-FUS group. | * In contrast to ALS-FUS, no genetic alterations of FUS have been reported to date for cases within the FTLD-FUS group. | ||
* 5–10% of all FTLD cases | |||
* Deposited Proteins: FUS, EWS, TAF-15. | |||
* FUS‐positive inclusions in FTLD cases show co‐aggregation of TAF15 and EWS | |||
**(Different from ALS-FUS) | |||
DDx (also FUS+ve): | DDx (also FUS+ve): | ||
*Spinocerebellar Ataxia (SCA) | *Spinocerebellar Ataxia (SCA) | ||
*Huntington Disease (SD) | *Huntington Disease (SD) | ||
==Atypical FTLD‐U== | |||
* Early onset frontotemporal dementia, rapidly progressive psycho‐behavioural changes. | |||
* Neuronal cytoplasmic inclusions in hippocampus and frontotemporal lobes. | |||
* Ubiquitin+ve, tau/TDP‐ve. | |||
* FET+ve inclusions | |||
** Unique vermiform filamentous neuronal nuclear inclusions. | |||
* Caudate nucleus head degeneration and hippocampal sclerosis. | |||
==Basophilic inclusion body disease== | ==Basophilic inclusion body disease== | ||
| Line 573: | Line 675: | ||
* Intraneuronal cytoplasmic basophilic inclusion bodies. | * Intraneuronal cytoplasmic basophilic inclusion bodies. | ||
* FUS+ve (universally). | * FUS+ve (universally). | ||
* TAF15+ve | * EWS+ve. | ||
* TAF15+ve. | |||
* alpha-Internexin+ve. | * alpha-Internexin+ve. | ||
==Neuronal Intermediate Filament Inclusion Disease== | ==Neuronal Intermediate Filament Inclusion Disease== | ||
* AKA: NIFID. | * AKA: NIFID. | ||
* Hyaline conglomerates (brightly eosinophilic branching fibrillar structures embedded in a round, well-delineated, glassy vacuole). | * Sporadic early‐onset frontotemporal dementia, motor neuron disease, extrapyramidal motor symptoms. | ||
* FUS+ve (heterogenous). | * Hyaline conglomerates (brightly eosinophilic branching fibrillar structures embedded in a round, well-delineated, glassy vacuole). | ||
* Deposits in cerebral cortex, hippocampus, basal ganglia, thalamus, cerebellar dentate, numerous brainstem nuclei and lower motor neurons. | |||
* FUS+ve/EWS+ve/TAF15+ve (heterogenous). | |||
** FET+ve filamentous nuclear inclusions in the hippocampus. | |||
* Ubiquitin +/-ve. | * Ubiquitin +/-ve. | ||
* NF +ve (some subunits). | * NF +ve (some subunits). | ||
| Line 646: | Line 752: | ||
==Amyotrophic lateral sclerosis== | ==Amyotrophic lateral sclerosis== | ||
*Abbreviated ''ALS''. | *Abbreviated ''ALS''. | ||
===General=== | ===General=== | ||
*[[AKA]] Lou Gehrig's disease. | *[[AKA]] Lou Gehrig's disease. | ||
*Characterized by motor neuron death. | *Characterized by motor neuron death. | ||
*May be familial and associated with ''SOD1'' | *May be familial and associated with ''C9orf72 expansion'', or ''SOD1'', ''FUS'' and ''TARDBP'' mutations.<ref name=Ref_PCPBoD8_679>{{Ref PCPBoD8|679}}</ref><ref>{{Cite journal | last1 = Guerrero | first1 = EN. | last2 = Wang | first2 = H. | last3 = Mitra | first3 = J. | last4 = Hegde | first4 = PM. | last5 = Stowell | first5 = SE. | last6 = Liachko | first6 = NF. | last7 = Kraemer | first7 = BC. | last8 = Garruto | first8 = RM. | last9 = Rao | first9 = KS. | title = TDP-43/FUS in motor neuron disease: Complexity and challenges. | journal = Prog Neurobiol | volume = 145-146 | issue = | pages = 78-97 | month = | year = | doi = 10.1016/j.pneurobio.2016.09.004 | PMID = 27693252 }}</ref> | ||
*Pathological protein aggregates cause dysfunction of RNA-binding proteins. | |||
Clinical: | ===Clinical=== | ||
*Weakness. | *Peak incidence: 50-60yrs. | ||
*2-5 per 100,000 individuals worldwide. | |||
*Dead after disease onset: Usu. 2-5yrs. | |||
*Weakness (Progressive bulbar, limb, thoracic, and abdominal muscle atrophy). | |||
*About 20% of ALS cases develop frontotemporal lobar degeneration (FTLD). | |||
*Environmental toxins are discussed (Guam ALS).<ref>{{Cite journal | last1 = Chernoff | first1 = N. | last2 = Hill | first2 = DJ. | last3 = Diggs | first3 = DL. | last4 = Faison | first4 = BD. | last5 = Francis | first5 = BM. | last6 = Lang | first6 = JR. | last7 = Larue | first7 = MM. | last8 = Le | first8 = TT. | last9 = Loftin | first9 = KA. | title = A critical review of the postulated role of the non-essential amino acid, β-N-methylamino-L-alanine, in neurodegenerative disease in humans. | journal = J Toxicol Environ Health B Crit Rev | volume = 20 | issue = 4 | pages = 1-47 | month = | year = 2017 | doi = 10.1080/10937404.2017.1297592 | PMID = 28598725 }}</ref> | |||
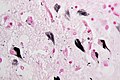
===Microscopic=== | ===Microscopic=== | ||
Features:<ref name=Ref_PCPBoD8_679>{{Ref PCPBoD8|679}}</ref> | Features:<ref name=Ref_PCPBoD8_679>{{Ref PCPBoD8|679}}</ref><ref>{{Cite journal | last1 = Saberi | first1 = S. | last2 = Stauffer | first2 = JE. | last3 = Schulte | first3 = DJ. | last4 = Ravits | first4 = J. | title = Neuropathology of Amyotrophic Lateral Sclerosis and Its Variants. | journal = Neurol Clin | volume = 33 | issue = 4 | pages = 855-76 | month = Nov | year = 2015 | doi = 10.1016/j.ncl.2015.07.012 | PMID = 26515626 }}</ref> | ||
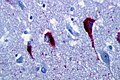
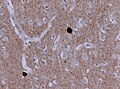
*Motor neurons with ''Bunina bodies''. | *Loss of the giant cells of Betz. | ||
*Motor neurons with eosinophilic inclusions (''Bunina bodies''). | |||
**PAS positive cytoplasmic inclusions. | **PAS positive cytoplasmic inclusions. | ||
*Motor neuron loss + reactive gliosis + neurogenic muscular atrophy. | *Motor neuron loss + reactive gliosis + neurogenic muscular atrophy. | ||
**Loss of myelinated axons in the lateral and anterior columns of the spinal cord. | |||
*Ubiquitinated cytoplasmic inclusions.<ref>{{Cite journal | last1 = Leigh | first1 = PN. | last2 = Anderton | first2 = BH. | last3 = Dodson | first3 = A. | last4 = Gallo | first4 = JM. | last5 = Swash | first5 = M. | last6 = Power | first6 = DM. | title = Ubiquitin deposits in anterior horn cells in motor neurone disease. | journal = Neurosci Lett | volume = 93 | issue = 2-3 | pages = 197-203 | month = Nov | year = 1988 | doi = | PMID = 2853844 }}</ref> | |||
*[[TDP-43]] proteinopathy in motor neurons (90% of all sporadic ALS cases). | |||
**SOD1-mutant cases are [[TDP-43]]-ve.<ref>{{Cite journal | last1 = Nakamura | first1 = S. | last2 = Wate | first2 = R. | last3 = Kaneko | first3 = S. | last4 = Ito | first4 = H. | last5 = Oki | first5 = M. | last6 = Tsuge | first6 = A. | last7 = Nagashima | first7 = M. | last8 = Asayama | first8 = S. | last9 = Fujita | first9 = K. | title = An autopsy case of sporadic amyotrophic lateral sclerosis associated with the I113T SOD1 mutation. | journal = Neuropathology | volume = 34 | issue = 1 | pages = 58-63 | month = Feb | year = 2014 | doi = 10.1111/neup.12049 | PMID = 23773010 }}</ref> | |||
*C9orf72 expansion cases: p62+ve, [[TDP-43]]-ve inclusions in the dentate gyrus, neocortex, and cerebellum.<ref>{{Cite journal | last1 = Al-Sarraj | first1 = S. | last2 = King | first2 = A. | last3 = Troakes | first3 = C. | last4 = Smith | first4 = B. | last5 = Maekawa | first5 = S. | last6 = Bodi | first6 = I. | last7 = Rogelj | first7 = B. | last8 = Al-Chalabi | first8 = A. | last9 = Hortobágyi | first9 = T. | title = p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. | journal = Acta Neuropathol | volume = 122 | issue = 6 | pages = 691-702 | month = Dec | year = 2011 | doi = 10.1007/s00401-011-0911-2 | PMID = 22101323 }}</ref> | |||
**FUS-mutant cases show FUS+ve, p62+ve (few) and [[TDP-43]]-ve inclusions.<ref>{{Cite journal | last1 = Vance | first1 = C. | last2 = Rogelj | first2 = B. | last3 = Hortobágyi | first3 = T. | last4 = De Vos | first4 = KJ. | last5 = Nishimura | first5 = AL. | last6 = Sreedharan | first6 = J. | last7 = Hu | first7 = X. | last8 = Smith | first8 = B. | last9 = Ruddy | first9 = D. | title = Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. | journal = Science | volume = 323 | issue = 5918 | pages = 1208-1211 | month = Feb | year = 2009 | doi = 10.1126/science.1165942 | PMID = 19251628 }}</ref> | |||
Images: | Images: | ||
| Line 665: | Line 784: | ||
*[http://pathology.mc.duke.edu/neuropath/CNSlecture4/alsbunina.jpg Bunina body (duke.edu)].<ref>URL: [http://pathology.mc.duke.edu/neuropath/CNSlecture4/CNSlecture4.htm http://pathology.mc.duke.edu/neuropath/CNSlecture4/CNSlecture4.htm]. Accessed on: 30 August 2011.</ref> | *[http://pathology.mc.duke.edu/neuropath/CNSlecture4/alsbunina.jpg Bunina body (duke.edu)].<ref>URL: [http://pathology.mc.duke.edu/neuropath/CNSlecture4/CNSlecture4.htm http://pathology.mc.duke.edu/neuropath/CNSlecture4/CNSlecture4.htm]. Accessed on: 30 August 2011.</ref> | ||
*[http://path.upmc.edu/cases/case291.html ALS - several images (upmc.edu)]. | *[http://path.upmc.edu/cases/case291.html ALS - several images (upmc.edu)]. | ||
<gallery> | |||
File:ALS-TDP-HE.jpg | Motor neuron loss in ALS-TDP. (WC) | |||
File:ALS-TDP-43_IHC.jpg | pTDP-43 positive inclusions in neurons. (WC) | |||
</gallery> | |||
DDx: | |||
*Spinal muscular atrophy. | |||
*Primary Lateral Sclerosis. | |||
*Hereditary Spastic Paraparesis (HSP). | |||
==Hallervorden-Spatz disease== | ==Hallervorden-Spatz disease== | ||
Latest revision as of 20:32, 24 May 2020
Neurodegenerative diseases is a big part of neuropathology. It includes some discussion of dementia.
Overview
- Neurodegenerative disease = essentially progressive and selective neuron loss.
- Clinically, they are not unique, e.g. dementia can be caused by several diseases (with different molecular etiologies).
- Each syndrome (e.g. dementia, parkinsonism, ataxia) has a most common etiology and a DDx.
- They are defined by molecular pathology.[1]
- The diseases are due to the accumulation of abnormal protein.
- The amino acid sequence of the protein may be completely normal. The problem may just be folding/protein conformation.
- The diseases are due to the accumulation of abnormal protein.
Molecular schema of neurodegenerative disorders:[1]
| Neurodegenerative disorders | |||||||||||||||||||||||||||||||||||||||||
| Amyloidoses | Tauopathies | α-synucleinopathies | TDP-43 | FUS/EWS/TAF15 | |||||||||||||||||||||||||||||||||||||
Common diseases
- Alzheimer disease (Abeta).
'Pure' tauopathies:
- Progressive supranuclear palsy.
- Pick's disease.
- Corticobasal degeneration.
- FTDP-17.
- Dementia pugilistica.
Synucleinopathies:[2]
- Parkinson disease.
- Dementia with Lewy bodies.
- Multiple system atrophy.
TDP-43 proteinopathies:
- Amyotrophic lateral sclerosis.
- Frontotemporal lobar degeneration with TDP-43 (FTLD-TDP).
FET proteinopathies:
- Basophilic inclusion body disease (BIBD).
- Neuronal intermediate filament inclusion disease (NIFID).
- Atypical frontotemporal lobar degeneration with ubiquitin-positive inclusions (atypical FTLD-U).
Prionopathies:
- Creutzfeldt-Jakob disease (PrP).
Note: Some people consider α-synuclein as a prion-like protein.[3]
Table
Disease/pathology/clinical correlation based on Dickson:[1]
| Disease | Deposited protein | Distribution | Clinical | Histology | Image |
|---|---|---|---|---|---|
| Alzheimer disease | Abeta (mutated APP) | corticolimbic, usu. spares occipital |
dementia | plaques, neurofibrillary tangles | [1] |
| Creutzfeldt-Jakob disease | PrPres (mutated PrP) | cortical & basal ganglia | dementia (rapid progression), movement disorder |
cytoplasmic vacuolization, PrP+ve plaques, Kuru plaques (MV2 variant) | [2] |
| Parkinson disease | alpha-synuclein | brainstem | parkinsonism | Lewy bodies in substantia nigra and locus coeruleus | [3] [4] |
| Dementia with Lewy bodies |
alpha-synuclein | corticolimbic, brainstem | dementia + parkinsonism | Lewy bodies brainstem and cortical, tangles | [5] [6] |
| Multiple system atrophy | alpha-synuclein | basal ganglia, brainstem, cerebellum | parkinsonism, ataxia | Papp-Lantos inclusions (cytoplasmic deposits in oligodendrocytes)[4] | [7] |
| Amyotrophic lateral sclerosis (ALS) |
TDP-43 | motor neurons | spasticity, weakness | motor neuron loss, TDP-43+ve, TAF15-ve, EWS-ve inclusions in motor neurons | [8] |
| Frontotemporal lobar degeneration with TDP-43 (FTLD-TDP) |
TDP-43 | cortex, basal ganglia | dementia, focal cortical syndromes | histology depends on (type 1-4), ubiquitin and TDP-43+ve, tau and FUS-ve | [9] |
| Frontotemporal lobar degeneration with FET (FTLD-FET) |
FUS/EWS/TAF15 | cortex, medulla, hippocampus, and motor cells of the spinal cord | dementia, cases classified as aFTLD-U, NIFID and BIBD | FUS+ve, TAF15+ve, EWS+ve cytoplasmic & intranuclear inclusions, neuritic threads | [10] |
| Progressive supranuclear palsy (FTLD-tau) | tau 4R | basal ganglia, brainstem | atypical parkinsonism with early gait instability, falls, and supranuclear gaze palsy | tau-positive globose neurofibrillary tangles in neurons, tufted astrocytes, coiled bodies in oligodendrocytes |
[11] |
| Pick disease (FTLD-tau) | tau 3R | corticolimbic | dementia + focal cortical syndrome |
Intraneuronal argyrophilic inclusions (Pick body) | [12] |
| Corticobasal degeneration (CBD) (FTLD-tau) | tau 4R | cortical, basal ganglia | dementia + movement disorder (Parkinson-plus syndrome) | ballooned neurons, astrocytic plaques, pretangles in basal nucleus | [13] |
| Argryophilic grain disease (AGD) (FTLD-tau) | tau 4R | medial temporal lobe, limbic structures | late-onset amnestic syndrome | Argyrophilic grains (also found unspecific in elederly) | [14] |
Immunohistochemistry
Alpha-synuclein
Look for:
- Lewy bodies (seen in Parkinson's Disease (PD), Dementia with Lewy bodies (DLB)) = round cytoplasmic eosinophilic body +/- pale halo.
- Lewy neurites(seen in PD and DLB) = abnormal neurites with filaments similar to those found in Lewy bodies.
- Glial cytoplasmatic inclusions (Papp-Lantos bodies) seen in mutisystem atrophy (MSA).
- Beta amyloid in vessels seen in cerebral amyloid angiopathy (CAA).
Tau
TDP-43
- May accumulate due to a progranulin mutation.
Microscopic
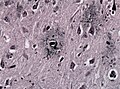
- TDP-43 - normally in the nucleus.
- Pathologic: Micrograph (label B) - neurites, skein-like formations (ama-assn.org)[7]
- Fibrillar or skein-like formations = cytoplasmic staining.
- "Skein" = yarn or thread wound on a reel or flock of birds in flight.[8]
- Neurites = "squiggly appearance"; "worm-like appearance".
- Fibrillar or skein-like formations = cytoplasmic staining.
- Pathologic: Micrograph (label B) - neurites, skein-like formations (ama-assn.org)[7]
Ubiquitin
- Marks proteins for recycling.
- Stains Barr bodies in hippocampal granule cells[9]
p62
- p62; poli-ubiquitin-binding protein p62.[5]
Microscopic
Look for:
- Lewy bodies and extracellular pigment in neuromelanin-containing nuclei (SN, LC, DVN) -> PD.
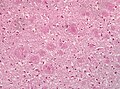
- Spongiform vacuolation in the neuropil (seen in Prion disease and FTLD-TDP).
- Neurofibrillar tangles (pyramidal layer of dentate gyrus).
- Granulovacuolar degeneration (granules within cytoplasmic vacuoles, mainly in the hippocampal pyramidal neurons, seen in AD).
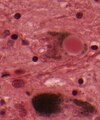
- Cores of amyloid plaqyes.
- Cotton wool plaques (seen in familiar AD).
- Pick cells (balloned neurons in frontal cortex).
- Pick bodies (granular layer of dentate gyrus).
- Extensive astrogliosis (striatonigral degeneration, hepatic encephalopathy).
- Corpora amylacea in the cornu ammonis may be increased in neurodegenerative diseases. [10]
Clinical perspective
- Correlations between clinical signs and molecular can be poor.
Dementia general (mostly useless) DDx
- Alzheimer's dementia - most common.
- Vascular.
- Multi-infarct dementia.
- Parkinson's associated dementia.
- Lewy body dementia.
- Alcohol-related dementia.
- Fronto-temporal dementia (Pick disease).
- Multisystem atrophy.
Mnemonic
Dementia mnemonic VITAMIN D VEST:[12]
- Vitamin deficiency (B12, folate, thiamine).
- Infection (HIV).
- Trauma.
- Anoxia.
- Metabolic (Diabetes).
- Intracranial tumour.
- Normal pressure hydrocephalus.
- Degenerative (Alzheimer's, Huntington's, CJD).
- Vascular.
- Endocrine.
- Space occupying lesion (chronic subdural hematoma).
- Toxins (alcohol).
Functional anatomy of dementia
- Hippocampus (essential for forming new memories).
- Frontal lobe (essential for retrieval of memories).
Parkinsonism causes
- Parkinson's disease [13]
- Dementia with Lewy bodies.
- Multiple system atrophy (MSA).[14]
- Progressive supranuclear palsy (PSP).[15]
- Drug induced (valproic acid, MPTP).[16][17]
- Vascular. [18]
- Postencephalitic. [19]
- Tramuatic (Dementia pugilistica).[20]
Amyloidoses
Alzheimer disease
General
- Onset: episodic memory loss.
- Diagnosis is clinical & pathologic.
- Pathologic finding alone are not diagnostic.
- Onset, rate of progression and the development of pathology are highly variable.
- Defined by:
- Pathological accumulation of amyloid β (Aβ) into extracellular plaques.
- Abnormally phosphorylated tau that accumulates intraneuronally forming neurofibrillary tangles (NFTs).
- Clinicopathological correlation better for NFT than for Aβ.[21]
- Seen in conjunction with vascular amyloid deposition; see cerebral amyloid angiopathy.
- Evidence of possible iatrogenic transmission by cadaver-sourced growth hormone batches.[22][23]
Genetics
Genes associated with Alzheimer disease:[24]
- Amyloid precursor protein (APP).
- On chromosome 21 - may explain why Trisomy 21 (Down syndrome) increases the risk of Alzheimer disease.[25]
- Presenilin 1 (PSEN1).[26]
- Presenilin 2 (PSEN2).[27]
- Apolipoprotein E (APOE)[28] - specifically the epsilon-4 allele.
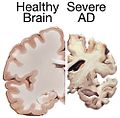
Gross
Features:
- Temporal atrophy, esp. hippocampus.
- Dilation of:
- Lateral ventricles.
- Third ventricle.
Gross/microscopic - disease spread by NF tangles (staging):[29]
- Alzheimer "spreads" in a reproducible pattern:
- Stage I-II: entorhinal cortex.
- Stage III-IV: inferior aspect of brain.
- Stage V-VI: limbic system.
Minimal sampling:
- Frontal, parietal & temporal lobe
- Hippocampus and entorhinal cortex
Additional sampling:
- Basal ganglia
- Cerebellum
- Midbrain (including substantia nigra)
- Occipital cortex
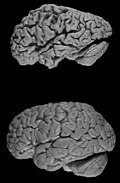
Images
Alzheimer's brain. (WC/NIH)
Microscopic
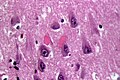
Features:
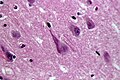
- Neurofibrillary tangles.
- Consists of tau.
- Location: hippocampus, cerebral cortex, hypothalamus.
- Dementia severity correlates better with NF tangles number than senile plaque number.[30]
- Six-tiered scoring method to assess tangle load [31]
- Images: tangles - schematic (pakmed.net)[32], tangle (washington.edu).[33]
- Senile plaques (AKA neuritic plaques).
- Consists of two components:
- Centre - radiates.
- Consists of Abeta amyloid
- Neurites - swollen axons.
- Centre - radiates.
- Considered to be more specific for Alzheimer's than NF tangles.
- How to remember: senile plaques = specific.
- There is a CERAD staging system for senile plaque load: 0 (none), I (mild), II (moderate), III (severe).[34]
- Images: senile plaques (utah.edu)[35] senile plaques - beta-APP - high mag. (WC).
- Consists of two components:
- Neuron loss.
- +/-Cerebral amyloid angiopathy.
Images
Classification
NIA/AA Guidelines: "ABC" scoring method [36]
- (A) assessment of amyloid b deposits
- (B) staging of neurofibrillary tangles
- (C) scoring of neuritic plaques
| (A) abeta plaques (Thal phase)[37] | (B) Neurofibrillary tangles (Braak stage) [38] | (C) neuritic plaques (CERAD) [39] |
|---|---|---|
| (A0) 0 | (B0) 0 | (C0) none |
| (A1) 1 (temporal),2 (+frontal, +CA1) | (B1) I,II (transentorhinal) | (C1) sparse (1–5 neuritic plaques/1 mm2) |
| (A2) 3 (+diencephalon, +striatum) | (B2) III,IV (limbic) | (C2) moderate(6–19 neuritic plaques/1 mm2) |
| (A3) 4 (+brainstem),5 (+cerebellum, +pons) | (B3) V,VI (neocortical) | (C3) frequent(>20 neuritic plaques/1 mm2) |
The ABC score is a good indicator for the likelihood of dementia.
Example: Cerebellar abeta deposits (A3) + tangles in entorhinal cortex and few temporal (B2), + 15 neuritic plaques per 1 mm2 (C2) -> (A3, B3, C2): intermediate AD level change.
Notes:
- Abeta amyloid:
- Derived from amyloid precursor protein (APP).
- APP:
- Rapid axonal transport - useful as a marker of axonal injury.
- Function currently not known.
- APP:
- Derived from amyloid precursor protein (APP).
- Tau:
- Important in microtubule assembly.
Prion diseases
General
Etiology:[40]
- Misfolded cell-surface protein called PrPSC.
- This is derived from the protein PrPC encoded by the PRNP gene.
- Different genetics strains are associated with varying clinical phenotype.[41]
Includes:
- Creutzfeldt-Jakob disease (CJD).
- Sporadic fatal insomnia (sFI).[40]
- Fatal familial insomnia (FFI).[42][43]
- Gestmann-Straussler-Scheinker syndrome (GSS) - due to PRNP gene mutations.[44]
IHC
PrPC:[42]
- Congo red +ve.
- PAS +ve.
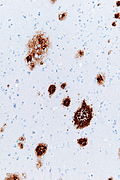
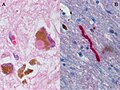
Creutzfeldt-Jakob disease
- Commonly abbreviated as CJD.
General
- Rare.
- Incurable disease.
Usually diagnosed clinically:
- Characteristic findings:
- Very rapid decline (3-4 months).
- Characteristic (cortex findings on) neuroradiology.
Variant Creutzfeldt-Jakob disease
- Abbreviated vCJD.
General
- Associated with bovine spongiform encephalopathy (AKA mad cow disease).
- Should sample: spleen, lymph nodes, tonsils.[45]
Microscopic
Features:
- Spongy appearance (cytoplasmic vacuolization[46]).
Note:
- Spongiform changes may be seen in ALS, Alzheimer's disease and Lewy body disease (e.g. Parkinson disease); however, the changes are only in the upper cortex and not diffuse.[47]
Molecular
- The CJD phenotype is associated with a PRNP D178N mutation and valine polymorphism at codon 129 (D178N-129V).
- Note: A Met129 polymorphism will cause Fatal familiar insomnia in the setting of the same PRNP D178N mutation. [48]
Alpha-synucleinopathies
Without clincial information Parkinson's disease and Dementia with Lewy bodies cannot separated in histology.
Dementia with Lewy bodies
General
Clinical features:
- Parkinsonian features.
- Hallucinations (visual).
- Progressive cognitive decline with fluctuations.
Microscopic
Features:
- Lewy bodies.
- Lewy neurites.
Note: Cortical Lewy bodies are easily missed in HE.
IHC
- Alpha-synuclein +ve.
Images
Parkinson disease
General
- Common - often sporadic.
- May be genetic.
Clinical TRAP:[49]
- Tremor.
- Rigidity.
- Akinesia.
- Postural instability.
Genetics:[50]
Gross
Features:[53]
- Abnormally pale substantia nigra.
- Pigmentation increases with age.
- Pale locus ceruleus.
Notes:
- Substantia nigra is a midbrain structure.
- Image: Midbrain - schematic (WC).
Microscopic
Features:[53]
- Loss of pigmented (catecholaminergic) neurons in the substantia nigra and locus ceruleus.
- Gliosis - due to neuron loss.
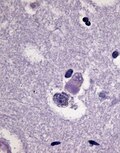
- Lewy bodies (in remaining neurons) - key feature.
- Eosinophilic cytoplasmic inclusion with "dense" (darker) core and pale (surrounding) halo.
- Consist of filaments composed of alpha-synuclein.
- Eosinophilic cytoplasmic inclusion with "dense" (darker) core and pale (surrounding) halo.
- Lewy neurites - alpha-synuclein positive processes.
IHC
- Alpha-synuclein +ve.
Images
Molecular
- Hereditary forms in less than 10% of the cases
- Involved genes are consecutively labeled PARK1, PARK2....
Multiple system atrophy
Multiple system atrophy is a neurodegenerative disease of the parkinsonism-plus disorder group.
General
Clinical findings variable:
- Parkinsonism (stiatonigral degeneration, MSA-P).
- Ataxia (olivo-ponto-cerebellar degeneration, MSA-C).
- Autonomic dysfunction (Shy-Drager syndrome, depreceated).
- Clinical onset between 40-60 years.
- Progedient tremor, atxia, laryngeal paresis, wakness, cognitive decline.
- Patients usually succumb after 6 years from aspiration pneumonia.
DDx:
- Spinocerebellar ataxia.
- Parkinson disease.
- Motor-neuron disease.
- Lewy-Body disease.
Macroscopy
- Cerebral (mild) & cerebellar atrophy.
- greenish putamen.
- Discoloration Substantia nigra and Locus coeruleus
Microscopic
Features:
- Inclusions cerebral, subcortical white matter, cerebellar.
- Neuronal loss and gliosis (absent in minimal-change MSA).
- Alpha-synuclein-rich glial and neuronal cytoplasmic inclusions in white matter (finding at autopsy).[54]
- Pons and Putamen:
- Nuclear inclusions (sparse in most cases).
- Neuropil threads (alpha-synuclein).
- Loss of myelinated fibers from external capsule, striatum and pallidum.
Images
Molecular
- No known alpha-synuclein mutation.
- Genetic variants of SNCA gene assoicated with MSA. [57]
Tauopathies
More than 20 different degenerative disorders can be classified as tauopathies.[58] FTLD-tau is an umbrella term used for tauopathies including PSP, CBD, PiD and GGT. [59]
Argyrophilic grain disease
Corticobasal degeneration
- AKA CBD.
- Symptoms may vary:
- Progressive asymmetrical rigidity and apraxia, progressive aphasia or dementia.
- Neuronal and glial Tau-positive inclusions.[60]
- Astrocytic plaques.
- Thread-like lesions and coiled bodies.
- Ballooned neurons +/-.
- Pathology is cortical and striatal and Gallyas-positive.
- Neuronal loss in the substantia nigra.
DD: PSP (widespread neurofibrillary degeneration, with characteristic globose NFT).
Globular glial tauopathies
- Commonly abbreviated GGT.
- AKA sporadic multiple system tauopathy.
- Rare disease.[61]
- Combination of frontotemporal dementia and motor neuron disease or only part thereof.
- 4-repeat tauopathy.
Microscopic
- Globular oligodendroglial and astrocytic Tau inclusions.
- Absence of tufted astrocytes.
- Mostly Gallyas-negative.
Progressive supranuclear palsy
- Commonly abbreviated PSP.
- AKA Steele-Richardson-Olszewski syndrome.
General
- Diagnosis - clinical.[62]
Clinical:
- Impaired control of gaze, esp. difficulty looking up and down (supranuclear palsy).[63]
- Parkinsonism.[15]
Microscopic
- Globose neurofibrillary tangles in neurons.
- Coiled bodies in oligodendrocytes.
- Wire coil-like structure around the nucleus.
- Tufted astrocytes.
- Near impossible to see without IHC - specifically AT8.
- Cellular processes filled with crap.
- Star-like appearance; looks like a road network where all the roads lead to one place (Parisian star).
- Grumose degeneration of the cerebellar dentate nucleus.
Images:
Pick disease
General
- Dementia.
Gross
Microscopic
Features:[67]
- Pick cells = large ballooned neurons.
- Pick bodies = round, homogenous, intracytoplasmic inclusions, size ~10 micrometers.
Image(s):
TDP Proteinopathies
FTLD-TDP
- Accounts for about 50% of all FTLD cases.
- Degeneration of frontal and temporal lobes.
- Inclusions not seen in HE or silver stains.
- TDP43-positive
- Neuronal cytoplasmic inclusions.
- Neuronal intranuclear inclusions.
- Dystrophic neurites.
- Ubiquitin+ve.
- p62+ve.
- aSynculein-ve.
- Tau-ve.
- FUS-ve.
- Four FTLD-TDP subtypes
- Type A: compact nuclear/cytoplasmatic inclusions, associated with GRN mutations.
- Type B: diffuse nuclear/cytoplasmatic inclusions most often seen in C9orf72 expansion.
- Type C: dystrophic neurites.
- Type D: Lentiform nuclear inclusions, only in cases with VCP mutations.
- C9orf72 mutated show additional DPR+ve staining of TDP‐43‐ve inclusions.
- These addtional inclusions are ubiquitin+ve and p62+ve
FTLD-FET
- Clinical manifestations depend on the distribution of the pathologic alterations in the CNS
- Currently 3 disorders among the FTLD-FET subgroup.
- In contrast to ALS-FUS, no genetic alterations of FUS have been reported to date for cases within the FTLD-FUS group.
- 5–10% of all FTLD cases
- Deposited Proteins: FUS, EWS, TAF-15.
- FUS‐positive inclusions in FTLD cases show co‐aggregation of TAF15 and EWS
- (Different from ALS-FUS)
DDx (also FUS+ve):
- Spinocerebellar Ataxia (SCA)
- Huntington Disease (SD)
Atypical FTLD‐U
- Early onset frontotemporal dementia, rapidly progressive psycho‐behavioural changes.
- Neuronal cytoplasmic inclusions in hippocampus and frontotemporal lobes.
- Ubiquitin+ve, tau/TDP‐ve.
- FET+ve inclusions
- Unique vermiform filamentous neuronal nuclear inclusions.
- Caudate nucleus head degeneration and hippocampal sclerosis.
Basophilic inclusion body disease
- AKA: BIBD.
- Variable clinic (behavioral, cognitive alterations, parkinsonism, motor neuron diseases, ALS-like).
- Age of onset: 35-70 years.
- Intraneuronal cytoplasmic basophilic inclusion bodies.
- FUS+ve (universally).
- EWS+ve.
- TAF15+ve.
- alpha-Internexin+ve.
Neuronal Intermediate Filament Inclusion Disease
- AKA: NIFID.
- Sporadic early‐onset frontotemporal dementia, motor neuron disease, extrapyramidal motor symptoms.
- Hyaline conglomerates (brightly eosinophilic branching fibrillar structures embedded in a round, well-delineated, glassy vacuole).
- Deposits in cerebral cortex, hippocampus, basal ganglia, thalamus, cerebellar dentate, numerous brainstem nuclei and lower motor neurons.
- FUS+ve/EWS+ve/TAF15+ve (heterogenous).
- FET+ve filamentous nuclear inclusions in the hippocampus.
- Ubiquitin +/-ve.
- NF +ve (some subunits).
- p62 +/-ve.
- TDP43-ve.
- Tau-ve.
- α-synuclein-ve.
Other
Chronic traumatic encephalopathy
- Abbreviated CTE.
Huntington disease
General
- Autosomal dominant inheritance.
- Mutation in Huntington gene (HTT):[70]
- 11-34 CAG repeat = normal.[71]
- >42 CAG repeat = Huntington disease.
Clinical:[72]
- Early onset dementia.
- Involuntary movements (chorea) - both arms and legs.
- Behaviour changes, e.g. grimacing.
- Speech changes.
Gross
Note:
- A normal caudate bulges into the ventricle.
Images:
Microscopic
Features:[72]
- Neuron loss.
- Gliosis.
Binswanger disease
General
- Multi-infarct dementia affecting subcortical white matter.
- Waste-basket diagnosis; diagnosed if CADASIL and amyloidosis have been excluded.
- Diagnosis has been controversial -- most with this entity (in the past) were diagnosed with Alzheimer's disease.
Microscopic
Features:
- Subcortical lesions that replace the myelin consisting of macrophages.
Frontotemporal lobar degeneration with ubiquitinated inclusions
Abbreviated FTLD with ubiquitinated inclusions or FTLD-TDP43.
General
- There are several forms of frontotemporal dementia.
- Related to amyotrophic lateral sclerosis (ALS); also a TDP-43 pathology.[75]
- There are several subtypes of FTLD with TDP-43.
Gross
- Frontal and temporal lobe atrophy.
Image:
Amyotrophic lateral sclerosis
- Abbreviated ALS.
General
- AKA Lou Gehrig's disease.
- Characterized by motor neuron death.
- May be familial and associated with C9orf72 expansion, or SOD1, FUS and TARDBP mutations.[76][77]
- Pathological protein aggregates cause dysfunction of RNA-binding proteins.
Clinical
- Peak incidence: 50-60yrs.
- 2-5 per 100,000 individuals worldwide.
- Dead after disease onset: Usu. 2-5yrs.
- Weakness (Progressive bulbar, limb, thoracic, and abdominal muscle atrophy).
- About 20% of ALS cases develop frontotemporal lobar degeneration (FTLD).
- Environmental toxins are discussed (Guam ALS).[78]
Microscopic
- Loss of the giant cells of Betz.
- Motor neurons with eosinophilic inclusions (Bunina bodies).
- PAS positive cytoplasmic inclusions.
- Motor neuron loss + reactive gliosis + neurogenic muscular atrophy.
- Loss of myelinated axons in the lateral and anterior columns of the spinal cord.
- Ubiquitinated cytoplasmic inclusions.[80]
- TDP-43 proteinopathy in motor neurons (90% of all sporadic ALS cases).
- C9orf72 expansion cases: p62+ve, TDP-43-ve inclusions in the dentate gyrus, neocortex, and cerebellum.[82]
Images:
DDx:
- Spinal muscular atrophy.
- Primary Lateral Sclerosis.
- Hereditary Spastic Paraparesis (HSP).
Hallervorden-Spatz disease
- AKA pantothenate kinase-associated neurodegeneration.
General
- Uncommon.
Microscopic
Features:[85]
- Axonal spheroids.
- Iron deposition.
Images:
Stains
- Prussian blue +ve.
See also
References
- ↑ 1.0 1.1 1.2 1.3 Dickson DW (2009). "Neuropathology of non-Alzheimer degenerative disorders". Int J Clin Exp Pathol 3 (1): 1–23. PMC 2776269. PMID 19918325. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2776269/?tool=pubmed.
- ↑ Uversky, VN. (Oct 2008). "Alpha-synuclein misfolding and neurodegenerative diseases.". Curr Protein Pept Sci 9 (5): 507-40. PMID 18855701.
- ↑ Watts, JC. (Oct 2019). "Calling α-synuclein a prion is scientifically justifiable.". Acta Neuropathol 138 (4): 505-508. doi:10.1007/s00401-019-02058-0. PMID 31407029.
- ↑ MUN. 15 November 2010.
- ↑ 5.0 5.1 Seelaar H, Klijnsma KY, de Koning I, et al. (May 2010). "Frequency of ubiquitin and FUS-positive, TDP-43-negative frontotemporal lobar degeneration". J. Neurol. 257 (5): 747–53. doi:10.1007/s00415-009-5404-z. PMC 2864899. PMID 19946779. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2864899/.
- ↑ Kumaran R, Kingsbury A, Coulter I, et al. (October 2007). "DJ-1 (PARK7) is associated with 3R and 4R tau neuronal and glial inclusions in neurodegenerative disorders". Neurobiol. Dis. 28 (1): 122–32. doi:10.1016/j.nbd.2007.07.012. PMID 17719794.
- ↑ Geser F, Brandmeir NJ, Kwong LK, et al. (May 2008). "Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis". Arch. Neurol. 65 (5): 636–41. doi:10.1001/archneur.65.5.636. PMID 18474740.
- ↑ URL: http://dictionary.reference.com/browse/skein. Accessed on: 20 November 2010.
- ↑ Gelpi, E.. "Clinical Neuropathology teaching case 3-2015: female or male brain? Anti-ubiquitin visualizes Barr bodies in hippocampal granule cells which allows the determination of gender in human brains.". Clin Neuropathol 34 (3): 115-6. PMID 25909954.
- ↑ Kovacs, GG.; Risser, D.. "Clinical Neuropathology image 6-2014: Corpora amylacea replacing cornu ammonis (CACA).". Clin Neuropathol 33 (6): 378-9. PMID 25343241.
- ↑ Coppola, G.; Chinnathambi, S.; Lee, JJ.; Dombroski, BA.; Baker, MC.; Soto-Ortolaza, AI.; Lee, SE.; Klein, E. et al. (Aug 2012). "Evidence for a role of the rare p.A152T variant in MAPT in increasing the risk for FTD-spectrum and Alzheimer's diseases.". Hum Mol Genet 21 (15): 3500-12. doi:10.1093/hmg/dds161. PMID 22556362.
- ↑ Shiau, Carolyn; Toren, Andrew (2006). Toronto Notes 2006: Comprehensive Medical Reference (Review for MCCQE 1 and USMLE Step 2) (22nd edition (2006) ed.). Toronto Notes for Medical Students, Inc.. pp. PS19. ISBN 978-0968592861.
- ↑ Tuite, PJ.; Krawczewski, K. (Apr 2007). "Parkinsonism: a review-of-systems approach to diagnosis.". Semin Neurol 27 (2): 113-22. doi:10.1055/s-2007-971174. PMID 17390256.
- ↑ Ahmed, Z.; Asi, YT.; Sailer, A.; Lees, AJ.; Houlden, H.; Revesz, T.; Holton, JL. (Nov 2011). "Review: The neuropathology, pathophysiology and genetics of multiple system atrophy.". Neuropathol Appl Neurobiol. doi:10.1111/j.1365-2990.2011.01234.x. PMID 22074330.
- ↑ 15.0 15.1 Bertram, K.; Williams, DR. (Apr 2012). "Visual hallucinations in the differential diagnosis of parkinsonism.". J Neurol Neurosurg Psychiatry 83 (4): 448-52. doi:10.1136/jnnp-2011-300980. PMID 22228724.
- ↑ Mahmoud, F.; Tampi, RR. (Oct 2011). "Valproic Acid-Induced Parkinsonism in the Elderly: A Comprehensive Review of the Literature.". Am J Geriatr Pharmacother. doi:10.1016/j.amjopharm.2011.09.002. PMID 21993183.
- ↑ Gerlach, M.; Riederer, P.; Przuntek, H.; Youdim, MB. (Dec 1991). "MPTP mechanisms of neurotoxicity and their implications for Parkinson's disease.". Eur J Pharmacol 208 (4): 273-86. PMID 1815982.
- ↑ Korczyn, AD. (Apr 2015). "Vascular parkinsonism-characteristics, pathogenesis and treatment.". Nat Rev Neurol. doi:10.1038/nrneurol.2015.61. PMID 25917706.
- ↑ Vilensky, JA.; Gilman, S.; McCall, S. (Jul 2010). "A historical analysis of the relationship between encephalitis lethargica and postencephalitic parkinsonism: a complex rather than a direct relationship.". Mov Disord 25 (9): 1116-23. doi:10.1002/mds.22908. PMID 20629120.
- ↑ Chauhan, NB. (2014). "Chronic neurodegenerative consequences of traumatic brain injury.". Restor Neurol Neurosci 32 (2): 337-65. doi:10.3233/RNN-130354. PMID 24398724.
- ↑ Nelson, PT.; Alafuzoff, I.; Bigio, EH.; Bouras, C.; Braak, H.; Cairns, NJ.; Castellani, RJ.; Crain, BJ. et al. (May 2012). "Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature.". J Neuropathol Exp Neurol 71 (5): 362-81. doi:10.1097/NEN.0b013e31825018f7. PMID 22487856.
- ↑ Duyckaerts, C.; Sazdovitch, V.; Ando, K.; Seilhean, D.; Privat, N.; Yilmaz, Z.; Peckeu, L.; Amar, E. et al. (Feb 2018). "Neuropathology of iatrogenic Creutzfeldt-Jakob disease and immunoassay of French cadaver-sourced growth hormone batches suggest possible transmission of tauopathy and long incubation periods for the transmission of Abeta pathology.". Acta Neuropathol 135 (2): 201-212. doi:10.1007/s00401-017-1791-x. PMID 29209767.
- ↑ Jaunmuktane, Z.; Mead, S.; Ellis, M.; Wadsworth, JD.; Nicoll, AJ.; Kenny, J.; Launchbury, F.; Linehan, J. et al. (Sep 2015). "Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy.". Nature 525 (7568): 247-50. doi:10.1038/nature15369. PMID 26354483.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 674-5. ISBN 978-1416054542.
- ↑ Nieuwenhuis-Mark, RE.. "Diagnosing Alzheimer's dementia in Down syndrome: problems and possible solutions.". Res Dev Disabil 30 (5): 827-38. doi:10.1016/j.ridd.2009.01.010. PMID 19269132.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 104311
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 600759
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 107741
- ↑ Braak H, Braak E, Bohl J (1993). "Staging of Alzheimer-related cortical destruction". Eur. Neurol. 33 (6): 403–8. PMID 8307060.
- ↑ Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; Aster, Jon (2009). Robbins and Cotran pathologic basis of disease (8th ed.). Elsevier Saunders. pp. 1317. ISBN 978-1416031215.
- ↑ Braak, H.; Braak, E. (1991). "Neuropathological stageing of Alzheimer-related changes.". Acta Neuropathol 82 (4): 239-59. PMID 1759558.
- ↑ URL: http://www.pakmed.net/academic/age/alz/alz030.htm. Accessed on: 12 November 2010.
- ↑ URL: http://faculty.washington.edu/alexbert/MEDEX/Fall/NeuroPath_Obj.htm. Accessed on: 13 November 2010.
- ↑ Mirra, SS.; Heyman, A.; McKeel, D.; Sumi, SM.; Crain, BJ.; Brownlee, LM.; Vogel, FS.; Hughes, JP. et al. (Apr 1991). "The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease.". Neurology 41 (4): 479-86. PMID 2011243.
- ↑ URL: http://library.med.utah.edu/WebPath/EXAM/IMGQUIZ/npfrm.html. Accessed on: 5 December 2010.
- ↑ Montine, TJ.; Phelps, CH.; Beach, TG.; Bigio, EH.; Cairns, NJ.; Dickson, DW.; Duyckaerts, C.; Frosch, MP. et al. (Jan 2012). "National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach.". Acta Neuropathol 123 (1): 1-11. doi:10.1007/s00401-011-0910-3. PMID 22101365.
- ↑ Thal, DR.; Rüb, U.; Orantes, M.; Braak, H. (Jun 2002). "Phases of A beta-deposition in the human brain and its relevance for the development of AD.". Neurology 58 (12): 1791-800. PMID 12084879.
- ↑ Braak, H.; Braak, E. (1991). "Neuropathological stageing of Alzheimer-related changes.". Acta Neuropathol 82 (4): 239-59. PMID 1759558.
- ↑ Mirra, SS.; Heyman, A.; McKeel, D.; Sumi, SM.; Crain, BJ.; Brownlee, LM.; Vogel, FS.; Hughes, JP. et al. (Apr 1991). "The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease.". Neurology 41 (4): 479-86. PMID 2011243.
- ↑ 40.0 40.1 Watts JC, Balachandran A, Westaway D (March 2006). "The expanding universe of prion diseases". PLoS Pathog. 2 (3): e26. doi:10.1371/journal.ppat.0020026. PMC 1434791. PMID 16609731. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1434791/.
- ↑ Monari, L.; Chen, SG.; Brown, P.; Parchi, P.; Petersen, RB.; Mikol, J.; Gray, F.; Cortelli, P. et al. (Mar 1994). "Fatal familial insomnia and familial Creutzfeldt-Jakob disease: different prion proteins determined by a DNA polymorphism.". Proc Natl Acad Sci U S A 91 (7): 2839-42. doi:10.1073/pnas.91.7.2839. PMID 7908444.
- ↑ 42.0 42.1 Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 672. ISBN 978-1416054542.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 600072
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 671. ISBN 978-1416054542.
- ↑ Burton, Julian L.; Rutty, Guy N. (2010). The Hospital Autopsy A Manual of Fundamental Autopsy Practice (3rd ed.). Oxford University Press. pp. 83. ISBN 978-0340965146.
- ↑ URL: http://moon.ouhsc.edu/kfung/jty1/opaq/PathQuiz/N0I002-PQ01-M.htm. Accessed on: 19 October 2010.
- ↑ Lefkowitch, Jay H. (2006). Anatomic Pathology Board Review (1st ed.). Saunders. pp. 419 Q4. ISBN 978-1416025887.
- ↑ Goldfarb, LG.; Petersen, RB.; Tabaton, M.; Brown, P.; LeBlanc, AC.; Montagna, P.; Cortelli, P.; Julien, J. et al. (Oct 1992). "Fatal familial insomnia and familial Creutzfeldt-Jakob disease: disease phenotype determined by a DNA polymorphism.". Science 258 (5083): 806-8. doi:10.1126/science.1439789. PMID 1439789.
- ↑ URL: http://www.nysslha.org/i4a/pages/index.cfm?pageid=3519. Accessed on: 30 March 2011.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 677. ISBN 978-1416054542.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 609007
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 602544
- ↑ 53.0 53.1 Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; Aster, Jon (2009). Robbins and Cotran pathologic basis of disease (8th ed.). Elsevier Saunders. pp. 1319. ISBN 978-1416031215.
- ↑ Wenning, GK.; Stefanova, N.; Jellinger, KA.; Poewe, W.; Schlossmacher, MG. (Sep 2008). "Multiple system atrophy: a primary oligodendrogliopathy.". Ann Neurol 64 (3): 239-46. doi:10.1002/ana.21465. PMID 18825660.
- ↑ MUN. 16 November 2010.
- ↑ Trojanowski JQ, Revesz T (2007). "Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy". Neuropathol. Appl. Neurobiol. 33 (6): 615–20. doi:10.1111/j.1365-2990.2007.00907.x. PMID 17990994.
- ↑ Pimenta, PF.; da Silva, RP.; Sacks, DL.; da Silva, PP. (Apr 1989). "Cell surface nanoanatomy of Leishmania major as revealed by fracture-flip. A surface meshwork of 44 nm fusiform filaments identifies infective developmental stage promastigotes.". Eur J Cell Biol 48 (2): 180-90. PMID 2743996.
- ↑ Williams, DR. (Oct 2006). "Tauopathies: classification and clinical update on neurodegenerative diseases associated with microtubule-associated protein tau.". Intern Med J 36 (10): 652-60. doi:10.1111/j.1445-5994.2006.01153.x. PMID 16958643.
- ↑ Forrest, SL.; Kril, JJ.; Stevens, CH.; Kwok, JB.; Hallupp, M.; Kim, WS.; Huang, Y.; McGinley, CV. et al. (Feb 2018). "Retiring the term FTDP-17 as MAPT mutations are genetic forms of sporadic frontotemporal tauopathies.". Brain 141 (2): 521-534. doi:10.1093/brain/awx328. PMID 29253099.
- ↑ Dickson, DW.; Bergeron, C.; Chin, SS.; Duyckaerts, C.; Horoupian, D.; Ikeda, K.; Jellinger, K.; Lantos, PL. et al. (Nov 2002). "Office of Rare Diseases neuropathologic criteria for corticobasal degeneration.". J Neuropathol Exp Neurol 61 (11): 935-46. PMID 12430710.
- ↑ Ahmed, Z.; Bigio, EH.; Budka, H.; Dickson, DW.; Ferrer, I.; Ghetti, B.; Giaccone, G.; Hatanpaa, KJ. et al. (Oct 2013). "Globular glial tauopathies (GGT): consensus recommendations.". Acta Neuropathol 126 (4): 537-544. doi:10.1007/s00401-013-1171-0. PMID 23995422.
- ↑ 62.0 62.1 URL: http://emedicine.medscape.com/article/1151430-overview. Accessed on: 11 November 2010.
- ↑ Levy, R. (Jun 2011). "[Progressive supranuclear palsy: what's new?].". Geriatr Psychol Neuropsychiatr Vieil 9 (2): 191-201. doi:10.1684/pnv.2011.0271. PMID 21690028.
- ↑ Williams DR, Lees AJ (March 2009). "Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges". Lancet Neurol 8 (3): 270–9. doi:10.1016/S1474-4422(09)70042-0. PMID 19233037.
- ↑ URL: http://neuropathologyblog.blogspot.com/2008/03/grumose-degeneration-in-cerebellar.html. Accessed on: 4 December 2010.
- ↑ Yamanouchi H, Yokoo H, Yuhara Y, et al. (March 2002). "An autopsy case of ornithine transcarbamylase deficiency". Brain Dev. 24 (2): 91–4. PMID 11891099.
- ↑ 67.0 67.1 Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 676. ISBN 978-1416054542.
- ↑ URL: http://medical-dictionary.thefreedictionary.com/Walnut+Brain. Accessed on: 14 March 2012.
- ↑ Grossman, M. (Feb 2010). "Primary progressive aphasia: clinicopathological correlations.". Nat Rev Neurol 6 (2): 88-97. doi:10.1038/nrneurol.2009.216. PMID 20139998.
- ↑ Kumar P, Kalonia H, Kumar A (2010). "Huntington's disease: pathogenesis to animal models". Pharmacol Rep 62 (1): 1–14. PMID 20360611.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 613004
- ↑ 72.0 72.1 Lefkowitch, Jay H. (2006). Anatomic Pathology Board Review (1st ed.). Saunders. pp. 415 Q44. ISBN 978-1416025887.
- ↑ URL: http://moon.ouhsc.edu/kfung/jty1/NeuroTest/Q07-Ans.htm. Accessed on: 29 October 2010.
- ↑ URL: http://path.upmc.edu/cases/case117/gross.html. Accessed on: 3 January 2012.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 105400
- ↑ 76.0 76.1 Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 679. ISBN 978-1416054542.
- ↑ Guerrero, EN.; Wang, H.; Mitra, J.; Hegde, PM.; Stowell, SE.; Liachko, NF.; Kraemer, BC.; Garruto, RM. et al. "TDP-43/FUS in motor neuron disease: Complexity and challenges.". Prog Neurobiol 145-146: 78-97. doi:10.1016/j.pneurobio.2016.09.004. PMID 27693252.
- ↑ Chernoff, N.; Hill, DJ.; Diggs, DL.; Faison, BD.; Francis, BM.; Lang, JR.; Larue, MM.; Le, TT. et al. (2017). "A critical review of the postulated role of the non-essential amino acid, β-N-methylamino-L-alanine, in neurodegenerative disease in humans.". J Toxicol Environ Health B Crit Rev 20 (4): 1-47. doi:10.1080/10937404.2017.1297592. PMID 28598725.
- ↑ Saberi, S.; Stauffer, JE.; Schulte, DJ.; Ravits, J. (Nov 2015). "Neuropathology of Amyotrophic Lateral Sclerosis and Its Variants.". Neurol Clin 33 (4): 855-76. doi:10.1016/j.ncl.2015.07.012. PMID 26515626.
- ↑ Leigh, PN.; Anderton, BH.; Dodson, A.; Gallo, JM.; Swash, M.; Power, DM. (Nov 1988). "Ubiquitin deposits in anterior horn cells in motor neurone disease.". Neurosci Lett 93 (2-3): 197-203. PMID 2853844.
- ↑ Nakamura, S.; Wate, R.; Kaneko, S.; Ito, H.; Oki, M.; Tsuge, A.; Nagashima, M.; Asayama, S. et al. (Feb 2014). "An autopsy case of sporadic amyotrophic lateral sclerosis associated with the I113T SOD1 mutation.". Neuropathology 34 (1): 58-63. doi:10.1111/neup.12049. PMID 23773010.
- ↑ Al-Sarraj, S.; King, A.; Troakes, C.; Smith, B.; Maekawa, S.; Bodi, I.; Rogelj, B.; Al-Chalabi, A. et al. (Dec 2011). "p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS.". Acta Neuropathol 122 (6): 691-702. doi:10.1007/s00401-011-0911-2. PMID 22101323.
- ↑ Vance, C.; Rogelj, B.; Hortobágyi, T.; De Vos, KJ.; Nishimura, AL.; Sreedharan, J.; Hu, X.; Smith, B. et al. (Feb 2009). "Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6.". Science 323 (5918): 1208-1211. doi:10.1126/science.1165942. PMID 19251628.
- ↑ URL: http://pathology.mc.duke.edu/neuropath/CNSlecture4/CNSlecture4.htm. Accessed on: 30 August 2011.
- ↑ URL: http://path.upmc.edu/cases/case207/dx.html. Accessed on: 11 January 2012.