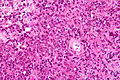
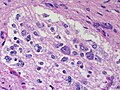
Difference between revisions of "Neuropathology tumours"
Jensflorian (talk | contribs) (→Infiltrative astrocytomas: All tumours listed in WHO 2007 category as astrocytoma) |
Jensflorian (talk | contribs) (sort + update ganglioglioma (needs split out in future)) |
||
| Line 250: | Line 250: | ||
==Pilocytic astrocytoma== | ==Pilocytic astrocytoma== | ||
{{Main|Pilocytic astrocytoma}} | {{Main|Pilocytic astrocytoma}} | ||
==Pilomyxoid astrocytoma== | |||
{{Main|Pilomyxoid astrocytoma}} | |||
==Pleomorphic xanthoastrocytoma== | ==Pleomorphic xanthoastrocytoma== | ||
*Abbreviated ''PXA''. | *Abbreviated ''PXA''. | ||
{{Main|Pleomorphic xanthoastrocytoma}} | {{Main|Pleomorphic xanthoastrocytoma}} | ||
==Subependymal giant cell astrocytoma== | ==Subependymal giant cell astrocytoma== | ||
| Line 263: | Line 262: | ||
{{Main|Subependymal giant cell astrocytoma}} | {{Main|Subependymal giant cell astrocytoma}} | ||
== | ==Oligoastrocytoma== | ||
{{Main| | {{Main|Oligoastrocytoma}} | ||
==Oligodendroglioma== | ==Oligodendroglioma== | ||
{{Main|Oligodendroglioma}} | {{Main|Oligodendroglioma}} | ||
== | ==Subependymoma== | ||
{{Main| | {{Main|Subependymoma}} | ||
== | ==Myxopapillary Ependymoma== | ||
{{Main| | {{Main|Myxopapillary Ependymoma}} | ||
==Ependymoma== | ==Ependymoma== | ||
{{Main|Ependymoma}} | {{Main|Ependymoma}} | ||
==Choroid plexus papilloma== | ==Choroid plexus papilloma== | ||
*Grade I WHO or Grade II WHO (atypical CPP) | |||
{{Main|Choroid plexus papilloma}} | {{Main|Choroid plexus papilloma}} | ||
| Line 315: | Line 284: | ||
{{Main|Choroid plexus carcinoma}} | {{Main|Choroid plexus carcinoma}} | ||
== | ==Angiocentric glioma== | ||
{{Main| | *Grade I WHO neuroepithelial tumour. | ||
{{Main|Angiocentric glioma}} | |||
==Chordoid glioma of the 3rd ventricle== | |||
* WHO grade II. | |||
* Slowly growing, non-invasive. | |||
* Clusters of epithelioid cells in mucinous stroma. | |||
* Lymphocytic infiltrates, adjacent Rosenthal fibers. | |||
* Few mitoses. | |||
* GFAP+ve, MIB-1 1-3%. | |||
==Gangliocytoma== | |||
* Grade I WHO neuronal tumour. | |||
** ICD-O code: 9492/0 | |||
* Groups of irregular large neurons. | |||
* Non-neoplastic, reticulin-rich glial stroma. | |||
==Ganglioglioma== | |||
:'''Not''' to be confused with ''[[ganglioneuroma]]''. | |||
===General=== | |||
*Grade I WHO mixed neuronal-glial tumour. | |||
*ICD-O code: 9505/1 (Anaplastic ganglioglioma: 9505/3) | |||
*Rare. | |||
*Usu. temporal lobe. | |||
*Recognized as a cause of [[epilepsy]].<ref name=pmid12125968>{{Cite journal | last1 = Im | first1 = SH. | last2 = Chung | first2 = CK. | last3 = Cho | first3 = BK. | last4 = Lee | first4 = SK. | title = Supratentorial ganglioglioma and epilepsy: postoperative seizure outcome. | journal = J Neurooncol | volume = 57 | issue = 1 | pages = 59-66 | month = Mar | year = 2002 | doi = | PMID = 12125968 }}</ref> | |||
===Microscopic=== | |||
Features: | |||
*Dysplastic neurons. | |||
**Out of regular architecture / abnormal location. | |||
**Cytomegaly | |||
**Clustering | |||
**Binucleated (very occassionally). | |||
*Atypical glia. | |||
*Calcification. | |||
*Lymphocytic cuffing. | |||
Anaplastic ganglioglioma: | |||
*Brisk mitotic activity | |||
*Necrosis | |||
===IHC=== | |||
*Neurons: | |||
**[[MAP2]] +ve | |||
**Synaptophysin +ve | |||
** Neurofilament +ve | |||
*Glia: | |||
**CD34+/-ve | |||
===DDx:=== | |||
*[[DNT]]. | |||
*[[Oligodendroglioma]]. | |||
*Trapped cortical neurons in diffuse astrocytoma. | |||
===Images=== | |||
<gallery> | |||
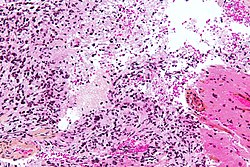
File:Ganglioglioma lymphocytic cuffing PAS.jpg | Lymphocytic cuffing in ganglioglioma (WC/jensflorian) | |||
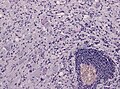
File:Ganglioglioma calcification.jpg | Calcification in ganglioglioma (WC/jensflorian) | |||
File:Ganglioglioma Cd34 x200.jpg | CD34 immunostain in ganglioglioma (WC/jensflorian) | |||
</gallery> | |||
*[http://path.upmc.edu/cases/case142.html Ganglioglioma - case 1 (upmc.edu)]. | |||
*[http://path.upmc.edu/cases/case282.html Ganglioglioma - case 2 (upmc.edu)]. | |||
==Dysembryoplastic neuroepithelial tumour== | |||
*Abbreviated ''DNT''. | |||
{{Main|Dysembryoplastic neuroepithelial tumour}} | |||
== | ==Atypical teratoid/rhabdoid tumour== | ||
{{Main| | :See also: ''[[Extrarenal malignant rhabdoid tumour]]''. | ||
*Commonly abbreviated ''AT/RT''. | |||
*May be written ''atypical teratoid rhabdoid tumour'', i.e. without the forward slash, or ''atypical teratoid-rhabdoid tumour'' (AT-RT). | |||
{{Main|Atypical teratoid/rhabdoid tumour}} | |||
==Medulloblastoma== | ==Medulloblastoma== | ||
| Line 346: | Line 383: | ||
</gallery> | </gallery> | ||
== | ==Meningioma== | ||
* | {{Main|Meningioma}} | ||
{{Main| | |||
==Peripheral nerve sheath tumours== | |||
{{Main|Peripheral nerve sheath tumours}} | |||
A classification:<ref name=pmid17893219>{{cite journal |author=Wippold FJ, Lubner M, Perrin RJ, Lämmle M, Perry A |title=Neuropathology for the neuroradiologist: Antoni A and Antoni B tissue patterns |journal=AJNR Am J Neuroradiol |volume=28 |issue=9 |pages=1633–8 |year=2007 |month=October |pmid=17893219 |doi=10.3174/ajnr.A0682 |url=http://www.ajnr.org/cgi/reprint/28/9/1633}}</ref> | |||
*Benign: | |||
**[[Schwannoma]]. | |||
**[[Neurofibroma]]. | |||
**[[Perineurioma]]. | |||
**[[Traumatic neuroma]]. | |||
*Malignant: | |||
**[[Malignant peripheral nerve sheath tumour]] (MPNST). | |||
==Schwannoma== | |||
{{Main|Schwannoma}} | |||
==Neurofibroma== | |||
{{Main|Neurofibroma}} | |||
==Ganglioneuroma== | |||
:'''Not''' to be confused with ''[[ganglioglioma]]''. | |||
*[[AKA]] ganglioma.<ref>URL: [http://medical-dictionary.thefreedictionary.com/ganglioma http://medical-dictionary.thefreedictionary.com/ganglioma]. Accessed on: 8 November 2010.</ref> | |||
{{Main|Ganglioneuroma}} | |||
==Chordoma== | |||
{{Main|Chordoma}} | |||
==Hemangioblastoma== | |||
{{Main|Hemangioblastoma}} | |||
==CNS lymphoma== | ==CNS lymphoma== | ||
| Line 407: | Line 464: | ||
{{Main|Central neurocytoma}} | {{Main|Central neurocytoma}} | ||
==Lhermitte-Duclos disease== | ==Lhermitte-Duclos disease== | ||
Revision as of 13:39, 13 October 2015
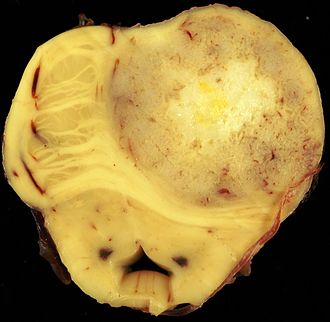
The article covers tumours in neuropathology. Tumours are a large part of neuropathology. Cytopathology of CNS tumours is dealt with in the article CNS cytopathology.
There are separate articles for peripheral nerve sheath tumours and pituitary/peri-pituitary lesions.
Brain tumours - overview
Adult
Four most common types of brain tumours:[1]
- Metastatic brain tumours (barely edges out primary tumours)
- Lung (most common).
- Breast.
- Melanoma.
- Renal cell carcinoma (RCC).
- Glioblastoma (previously known as glioblastoma multiforme).
- Anaplastic astrocytoma.
- Meningioma.
Children
- Pilocytic astrocytoma.
- Medulloblastoma.
- Ependymoma.
Location (most common)
Certain tumours like to hang-out at certain places:[2]
- Cerebrum:
- Cortical based - oligodendroglioma.
- Grey-white junction - metastases.
- White matter - astrocytoma, glioblastoma.
- Periventricular - CNS lymphoma.
- Cystic - ganglioglioma, pilocytic astrocytoma, pleomorphic xanthoastrocytoma.
- Cerebellum:
- Midline/central - medulloblastoma.
- Cystic lesion - pilocytic astrocytoma (younger individual), hemangioblastoma (older individual).
- Solid lesion (older individual) - metastasis.
- Spinal cord:
- Ependymoma, glioblastoma.
- Filum terminale - myxopapillary ependymoma, paraganglioma.
Filum terminale
- Filum terminale = bottom end of the spinal cord - has a limited differential.
DDx:[3]
Cerebellopontine angle
- Abbreviated CP angle.
DDx:[4]
- Schwannoma.
- Meningioma.
- Dermoid cyst/epidermoid cyst.
- Ependymoma.
- Choroid plexus papilloma.
Cystic tumours
DDx:[5]
- Pilocytic astrocytoma.
- Pleomorphic xanthoastrocytoma.
- Ganglioglioma.
- Hemangioblastoma.
- Craniopharyngioma.[6]
Primary versus secondary
- AKA (primary) brain tumour versus metastatic cancer.
Primary
Glial tumours:
- Cytoplasmic processes - key feature.
- Best seen at highest magnification - usu. ~1 micrometer.
- Processes may branch.
- Ill-defined border/blend with the surrounding brain.
- Large (lymphoid) cells, ergo usu. not a difficult diagnosis.
- ~2x size of resting lymphocyte, nucleoli.
- Lesion predominantly perivascular.
Secondary
Carcinomas:
- Well-demarcated border between brain and lesion - key feature.
- No cytoplasmic processes.
- Usu. have nuclear atypia of malignancy.
- Nuclei often ~3-4x the size of a RBC.
- +/-Glandular arrangement.
- +/-Nucleoli.
Common neuropathology tumours in a table
| Type | Key feature(s) | Imaging | History | Notes | IHC | Images |
| Normal tissue | cells regularly spaced, no nuc. atypia | small lesion? / deep lesion? | variable | missed lesion? | nil | |
| Reactive astrocytes | astrocytes with well-demarcated eosinophilic cytoplasm, regular spacing, no nuc. atypia | small lesion? / deep lesion? | variable | missed lesion / close to a lesion; non-specific pathologic process - need more tissue | nil | |
| Schwannoma | cellular areas (Antoni A), paucicelluar areas (Antoni B), palisading of nuclei (Verocay bodies) | extra-axial + intradural | old or young | need frozen section to Dx, DDx: meningioma | S100 | |
| Meningioma | whorls, psammomatous calcs, nuclear inclusions | extra-axial + intradural | old or young | may be diagnosed on smear, DDx: schwannoma, choroid plexus | EMA, PR, Ki-67 | |
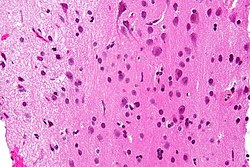
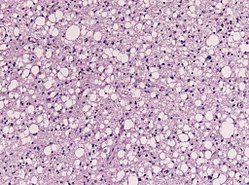
| Infiltrative astrocytoma (WHO grade II or grade III) | glial processes (esp. on smear), nuclear atypia (typical size var. ~3x, irreg. nuc. membrane, hyperchromasia), no Rosenthal fibres in the core of the lesion †, no microvascular proliferation, no necrosis | often enhancing (suggests high grade), usu. supratentorial, usu. white matter | usu. old, occ. young | common | IDH-1+/-, GFAP+ | |
| Glioblastoma (WHO grade IV) | glial processes (esp. on smear), nuclear atypia (typical size var. ~3x, irreg. nuc. membrane, hyperchromasia), no Rosenthal fibres in the core of the lesion †, microvascular proliferation or necrosis | often enhancing (suggests high grade), usu. supratentorial, usu. white matter | usu. old, occ. young | very common, esp. glioblastoma | IDH-1+/-, GFAP+ | |
| Metastasis | sharp interface with brain, often glandular, +/-nucleoli, no glial processes | often cerebellular, well-circumscribed | usu. old | often suspected to have metastatic disease | TTF-1, CK7, CK20, BRST-2 |
† Rosenthal fibres at the periphery of a lesion are a non-specific finding seen in chronic processes.
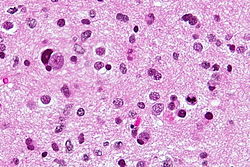
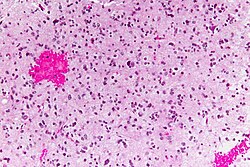
Brain metastasis
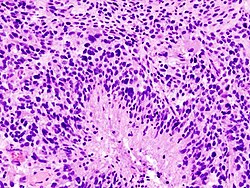
Infiltrative astrocytomas
Overview
- Low-grade (diffuse) astrocytomas (WHO Grade II).
- Anaplastic astrocytomas (WHO Grade III).
- Glioblastoma(WHO Grade IV).
- Gliosarcoma (WHO Grade IV).
- Gliomatosis cerebri (Grade III/IV).
Notes:
- Non-infiltrative astrocytomas:
- Pilocytic astrocytoma (WHO Grade I).
- Pilomyxoid astrocytoma (WHO Grade II).
- Pleomorphic xanthoastrocytoma (WHO grade II).
- Subependymal giant cell astrocytoma (WHO grade I).
- Pilocytic astrocytoma (WHO Grade I).
Microscopic
- Glial processes - key feature.
- Thin stringy cytoplasmic processes - best seen at high power in less cellular areas.
- No Rosenthal fibres within the tumour itself.
Images:
- Endothelial proliferation in a GBM (ouhsc.edu).
- Endothelial proliferation (ouhse.edu).
- Gemistocytic astrocytoma - several images (upmc.edu).
Notes:
- Glial vs. non-glial tumours:
- Glial: "blends into brain"/gradual transition to non-tumour brain.
- Non-glial: no glial processes.
- Rosenthal fibres within the tumour... make it into a pilocytic astrocytoma.
- Rosenthal fibres may be seen around a (very) slow growing tumour and represent a reactive process.
- Inflammatory cells and macrophages should prompt consideration of an alternate diagnosis (e.g. cerebral infarct, multiple sclerosis) - esp. if this is a primary lesion.[9]
Grading
Nuclear pleomorphism present:
- At least grade II (diffuse astrocytoma).
Mitotic figures present:
- At least grade III (anaplastic astrocytoma).
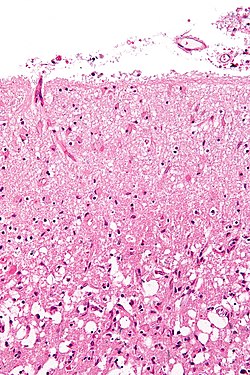
Microvascular proliferation or necrosis with pseudopalisading tumour cells:
- Grade IV (glioblastoma AKA glioblastoma multiforme).
Notes:
- Pseudopalisading tumour cells = high tumour cell density adjacent to regions of necrosis; palisade = a fence of poles forming a defensive barrier or fortification.
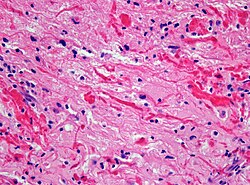
Images
Glioblastoma:
Anaplastic astrocytoma:
Table of common gliomas - grading
Histomorphologic comparison of common gliomas:
| Entity | Rosenthal fibres / EGBs |
Nuclear atypia | Mitoses | Necrosis or MVP | Infiltrative | Image |
| Pilocytic astrocytoma | yes | usu. no | usu. no | usu. no | no | |
| Low-grade astrocytoma | no | yes | no | no | yes | |
| Anaplastic astrocytoma | no | yes | yes | no | yes | |
| Glioblastoma | no | yes | yes | yes | yes |
Notes:
- MVP = microvascular proliferation.
- EGBs = eosinophilic granular bodies.
IHC
- GFAP - should stain cytoplasm of tumour cells and the perikaryon (nuclear membrane).
- Ki-67 - usu. high >20% of cells.
- p53 - often +ve.
- IDH1 (isocitrate dehydrogenase 1).
- +ve in tumours that arose from low-grade gliomas.[10]
- Image: IDH1 +ve in glioblastoma (WP).
- +ve in tumours that arose from low-grade gliomas.[10]
Notes:
- IDH1 and IDH2 mutations - better survival.[11]
Pilocytic astrocytoma
Pilomyxoid astrocytoma
Pleomorphic xanthoastrocytoma
- Abbreviated PXA.
Subependymal giant cell astrocytoma
- Abbreviated SEGA.
Oligoastrocytoma
Oligodendroglioma
Subependymoma
Myxopapillary Ependymoma
Ependymoma
Choroid plexus papilloma
- Grade I WHO or Grade II WHO (atypical CPP)
Choroid plexus carcinoma
Angiocentric glioma
- Grade I WHO neuroepithelial tumour.
Chordoid glioma of the 3rd ventricle
- WHO grade II.
- Slowly growing, non-invasive.
- Clusters of epithelioid cells in mucinous stroma.
- Lymphocytic infiltrates, adjacent Rosenthal fibers.
- Few mitoses.
- GFAP+ve, MIB-1 1-3%.
Gangliocytoma
- Grade I WHO neuronal tumour.
- ICD-O code: 9492/0
- Groups of irregular large neurons.
- Non-neoplastic, reticulin-rich glial stroma.
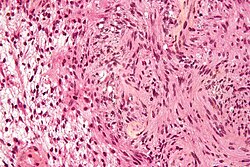
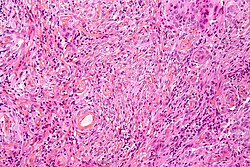
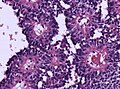
Ganglioglioma
- Not to be confused with ganglioneuroma.
General
- Grade I WHO mixed neuronal-glial tumour.
- ICD-O code: 9505/1 (Anaplastic ganglioglioma: 9505/3)
- Rare.
- Usu. temporal lobe.
- Recognized as a cause of epilepsy.[12]
Microscopic
Features:
- Dysplastic neurons.
- Out of regular architecture / abnormal location.
- Cytomegaly
- Clustering
- Binucleated (very occassionally).
- Atypical glia.
- Calcification.
- Lymphocytic cuffing.
Anaplastic ganglioglioma:
- Brisk mitotic activity
- Necrosis
IHC
- Neurons:
- MAP2 +ve
- Synaptophysin +ve
- Neurofilament +ve
- Glia:
- CD34+/-ve
DDx:
- DNT.
- Oligodendroglioma.
- Trapped cortical neurons in diffuse astrocytoma.
Images
Dysembryoplastic neuroepithelial tumour
- Abbreviated DNT.
Atypical teratoid/rhabdoid tumour
- See also: Extrarenal malignant rhabdoid tumour.
- Commonly abbreviated AT/RT.
- May be written atypical teratoid rhabdoid tumour, i.e. without the forward slash, or atypical teratoid-rhabdoid tumour (AT-RT).
Medulloblastoma
- Tumour of cerebellum - key feature.
- Morphologically identical supratentorial tumours are called primitive neuroectodermal tumour (PNET).
Primitive neuroectodermal tumour
Embryonal tumour with abundant neuropil and true rosettes
- Abbreviated ETANTR.
Astroblastoma
- No WHO grade yet.
- Very rare superficial tumor of young age.
- Large, cystic. Pushing margin towards CNS.
- Vasocentric growth, plump cells with absence of fibrillary pattern.
- GFAP+ve, Synaptohysin-ve, focally EMA/panCK+ve. MIB-1: 1-18 %.
Meningioma
Peripheral nerve sheath tumours
A classification:[13]
- Benign:
- Malignant:
Schwannoma
Neurofibroma
Ganglioneuroma
- Not to be confused with ganglioglioma.
Chordoma
Hemangioblastoma
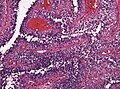
CNS lymphoma
Classification:
- Primary CNS lymphoma.
- Non-primary CNS lymphoma - see lymphoma article.
General - primary CNS
- Classically periventicular distribution.
- Usually large B cell; can be considered a type of diffuse large B cell lymphoma (DLBCL).
- Prognosis of CNS (DLBCL) lymphomas worse than nodal (non-CNS) DLBCL.[15]
Microscopic
Features:
- Large cell lymphoma.
- Size = 2x diameter normal lymphocyte.
- Nucleolus - common.
- Perivascular clustering.
Images
www:
IHC
Can be subclassified in GCB (germinal centre B-cell-like) and non-GCB by CD10, Bcl-6, MUM1/IRF-4, and Bcl-2.[15]
Common pattern:
- CD20 +ve - key stain.
- CD3 -ve.
- Ki-67 ~40%.
- Bcl-6 +ve.
- Bcl-1 -ve.
Neurocytoma
Central neurocytoma
- Abbreviated CNC.
Lhermitte-Duclos disease
- Abbreviated LDD.
- AKA dysplastic cerebellar gangliocytoma.[16]
- AKA dysplastic gangliocytoma of the cerebellum.
Ganglioneuroblastoma
General
- Uncommon.
- Part of the neuroblastic tumours group which includes:[17]
- Ganglioneuroma (benign).
- Ganglioneuroblastoma (intermediate).
- Neuroblastoma (aggressive).
Microscopic
Features:
- Ganglion-like cells with a prominent nucleolus.
- Small undifferentiated cells with scant cytoplasm.
Images:
IHC
- NSE +ve -- small cells.
Lesions of the sella turcica
Lesions of the sella turcica, the pituitary gland environs, is a topic for it self. The differential diagnosis for lesions in this area includes:
- Pituitary adenoma.
- Craniopharyngioma.
- Rathke cleft cyst.
- Germ cell tumour.
- Meningioma.
- Pilomyxoid astrocytoma - in children.
See also
References
- ↑ http://neurosurgery.mgh.harvard.edu/abta/primer.htm
- ↑ URL: http://www.msdlatinamerica.com/ebooks/DiagnosticNeuropathologySmears/files/4ce563fb7e8e48fc9ed8b42e296a7747.gif and http://www.msdlatinamerica.com/ebooks/DiagnosticNeuropathologySmears/sid117213.html. Accessed on: 2 November 2010.
- ↑ JLK. 31 May 2010.
- ↑ R. Kiehl. 8 November 2010.
- ↑ URL: http://path.upmc.edu/cases/case320/dx.html. Accessed on: 14 January 2012.
- ↑ URL: http://www.pathologyoutlines.com/Cnstumor.html#cystsgeneral. Accessed on: 14 January 2012.
- ↑ Rong Y, Durden DL, Van Meir EG, Brat DJ (June 2006). "'Pseudopalisading' necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis". J. Neuropathol. Exp. Neurol. 65 (6): 529–39. PMID 16783163.
- ↑ http://dictionary.reference.com/browse/palisading
- ↑ URL: http://path.upmc.edu/cases/case79/dx.html. Accessed on: 2 January 2012.
- ↑ Yan H, Parsons DW, Jin G, et al. (February 2009). "IDH1 and IDH2 mutations in gliomas". N. Engl. J. Med. 360 (8): 765–73. doi:10.1056/NEJMoa0808710. PMC 2820383. PMID 19228619. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2820383/.
- ↑ Houillier C, Wang X, Kaloshi G, et al. (October 2010). "IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas". Neurology 75 (17): 1560–6. doi:10.1212/WNL.0b013e3181f96282. PMID 20975057.
- ↑ Im, SH.; Chung, CK.; Cho, BK.; Lee, SK. (Mar 2002). "Supratentorial ganglioglioma and epilepsy: postoperative seizure outcome.". J Neurooncol 57 (1): 59-66. PMID 12125968.
- ↑ Wippold FJ, Lubner M, Perrin RJ, Lämmle M, Perry A (October 2007). "Neuropathology for the neuroradiologist: Antoni A and Antoni B tissue patterns". AJNR Am J Neuroradiol 28 (9): 1633–8. doi:10.3174/ajnr.A0682. PMID 17893219. http://www.ajnr.org/cgi/reprint/28/9/1633.
- ↑ URL: http://medical-dictionary.thefreedictionary.com/ganglioma. Accessed on: 8 November 2010.
- ↑ 15.0 15.1 Raoux D, Duband S, Forest F, et al. (June 2010). "Primary central nervous system lymphoma: Immunohistochemical profile and prognostic significance". Neuropathology 30 (3): 232–40. doi:10.1111/j.1440-1789.2009.01074.x. PMID 19925562.
- ↑ Yağci-Küpeli, B.; Oguz, KK.; Bilen, MA.; Yalçin, B.; Akalan, N.; Büyükpamukçu, M. (Mar 2010). "An unusual cause of posterior fossa mass: Lhermitte-Duclos disease.". J Neurol Sci 290 (1-2): 138-41. doi:10.1016/j.jns.2009.12.010. PMID 20060133.
- ↑ Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B (July 1999). "Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee". Cancer 86 (2): 349–63. PMID 10421272.